A Running List Of Covid-19 Treatments In Development
Disclaimer: This post is not medical advice, it is only for informational purposes. In case of need, consult your doctor at all times to make decisions about your health.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a positive sense, single-stranded RNA beta coronavirus, a member of Beta-CoV lineage B (subgenus Sarbecovirus).
COVID-19 represents a global health threat and it is a serious driver of possible economic crisis, so curbing the current ongoing outbreak is the matter of top priority for most health organizations and biotech companies.
In order to follow the quick pace of progress in the area of coronavirus treatments, we decided to create and maintain a list of key programs and projects, with data about companies and specific clinical trials where available. Suggest missing programs and molecules in the comments below.
The majority of molecules in the below list represent the case of drug repurposing when a molecule has prior known activity against some other indication. Drug repurposing is the most efficient option to come up with a new cure quickly -- a lot of safety data and other study results are already available for the molecule which allows to substantially accelerate the drug development process.
Most of the potential treatments against the coronavirus Covid-19 fall within two categories: treatments for the respiratory symptoms, and inhibitors of viral growth.
Table1. A Running List Of Covid-19 Treatments In Development (Antivirals)
| Candidate | MoA / indication | Status/clinical trials | Sponsor/Producer |
|---|---|---|---|
| Kaletra (lopinavir/ritonavir) Combinational therapy |
HIV protease inhibitor HIV-1 infection |
> 10 latest stages clinical studies (Including combinations with other drugs) NCT04321174 NCT04255017 NCT04307693 |
AbbVie |
| COVID-19 antibody therapy | antibody | Development stage | AbCellera Eli Lilly |
LAM-002A (Apilimod) |
Block cellular entry and trafficking of the SARS-CoV-2 virus Autoimmune diseases and follicular lymphoma |
Phase II clinical trials NCT04446377 |
AI Therapeutics, Yale University |
| AN01 (rhsACE2) Recombinant human angiotensin-converting enzyme 2 |
Blocks the spike protein, can protect the lung, blood vessels or the heart from injury via its enzyme function. | Phase II clinical trials NCT04335136 |
Apeiron Biologics |
| TMC-310911 (ASC-09) |
Novel investigational protease inhibitor (structurally similar to darunavir) | Clinical Studies of Combinational Therapies NCT04261907 NCT04261270 |
Ascletis, First Affiliated Hospital of Zhejiang University, Tongji Hospital |
Ganovo (Danoprevir) |
Hepatitis C virus protease inhibitor Hepatitis C |
Phase 4 Clinical Study In combination with other drugs NCT04291729 |
Ascletis, The Ninth Hospital of Nanchang |
| BGE-175 | a potent oral inhibitor ofthe prostaglandin D2 (PGD2) DP1 pathway | Phase 2 NCT04705597 |
BioAge Labs, Inc. |
| HFB30132A | Monoclonal Antibody Directed Against SARS-CoV-2, | Phase 1 NCT04590430 |
HiFiBiO Therapeutics |
| Itolizumab (Alzumab) Humanized IgG1 monoclonal antibody |
Inhibits CD6 to shut down the activation and trafficking of pathogenic T cells that drive auto-immune response. Indication: Chronic plaque psoriasis |
Completed Phase II clinical trials in India. NCT04475588 |
Equillium, Biocon |
Galidesivir (BCX4430)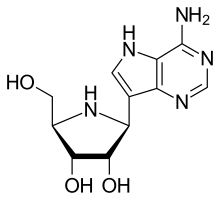 |
Nucleoside RNA polymerase inhibitor Yellow Fever |
Advanced development stage | BioCryst Pharmaceuticals |
Bemcentinib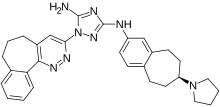 |
AXL kinase inhibitor. Indication: therapy resistant cancers. |
Phase II Clinical Trials 2020-001736-95 |
BerGenBio University Hospital Southampton NHS Foundation Trust, UK |
| BOLD-100 | Inhibit stress-induced upregulation of GRP78 Cancer drug |
Suggested | Bold Therapeutics |
| Leronlimab (PRO 140) Humanized monoclonal antibody |
Binds to CCR5 HIV, cancer |
Initiation of Phase 2 Clinical Study | CytoDyn Inc. |
Ivermectin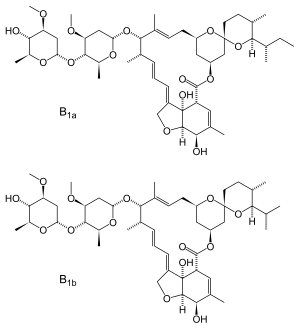 |
Anti-parasitic drug | Preclinical study |
Doherty Institute Monash University in Australia |
Fingolimod |
Sphingosine 1-phosphate receptor modulator Multiple sclerosis |
Phase 2 Clinical Study in China NCT04280588 |
First Affiliated Hospital of Fujian Medical University |
Thalidomide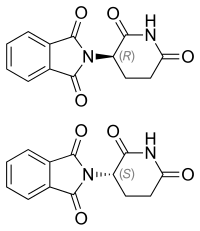 |
MoA is not fully understood | Phase 2 Clinical Trials in China NCT04273581 NCT04273529 |
First Affiliated Hospital of Wenzhou Medical University |
Remdesivir (GS - 5734) |
Block RNA polymerase Ebola |
Orphan Drug Designation for Gilead 9 clinical studies worldwide NCT04323761 NCT04257656 NCT04315948 |
Gilead Sciences |
| Truvada (emtricitabine + tenofovir) Combinational therapy |
Non-nucleoside reverse transcriptase inhibitors HIV infection |
In preparation | Gilead Sciences |
Triazavirin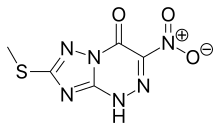 |
inhibits RNA synthesis | Phase 3 Clinical Study in China ChiCTR2000030001 |
Health commission of Heilongjiang province |
|
Baricitinib COV-BARRIER (LY3009104) baricitinib |
JAK/NAK inhibitor
COVID-19
|
Phase 3 Clinical Study in Italy
Phase 3 Clinical Study |
Hospital of Prato
|
| Prezista/ Prezcobix (darunavir + cobicistat) Combinational therapy |
Protease inhibitor HIV infection |
Phase 3 Clinical Studies NCT04304053 in Spain 3Cclinical tStudies in China NCT04252274 ChiCTR2000030259 ChiCTR2000029541 |
Janssen Pharmaceuticals Fundacio Lluita Contra la SIDA, Medical Institutions in China |
Chloroquine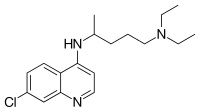 |
Endosomal acidification fusion inhibitor Anti-malarial |
> 10 studies worldwide > 10 Clinical Studies in China ChiCTR2000029609 NCT04261517 |
Medical institutions worldwide |
Azithromycin |
Antibiotic | > 10 trials in combination with other drugs NCT04322396 NCT04321278 NCT04322123 |
Medical institutions worldwide |
| Remestemcel-L Mesenchymal stromal cell (MSC) |
Migrate to the site of inflammation to reduce the production of pro-inflammatory cytokines. Indication: Acute Graft versus Host Disease |
Phase III Clinical Trials NCT04371393 |
Mesoblast, Inc. / Icahn School of Medicine at Mount Sinai |
| Aviptadil (RLF-100) Vasoactive Intestinal Polypeptide VIP |
Block replication of the SARS-CoV-2 virus in human lung cells and monocytes | Phase 2/3 Clinical Trials NCT04311697 NCT04360096 NCT04536350 |
NeuroRx, Relief Therapeutics |
Favipiravir (T-705)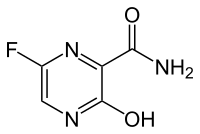 |
Block RNA polymerase Flu drug |
Approved in China Clinical studies in China, Japan, and Italy ChiCTR2000029996 ChiCTR2000030894 |
Produced by Fujifilm Toyama Chemical |
| Kevzara (sarilumab) Monoclonal antibody |
Anti - IL-6 Rheumatoid arthritis |
Phase 2, 3 Clinical Study NCT04315298 |
Regeneron Pharmaceuticals, Sanofi |
EIDD-2801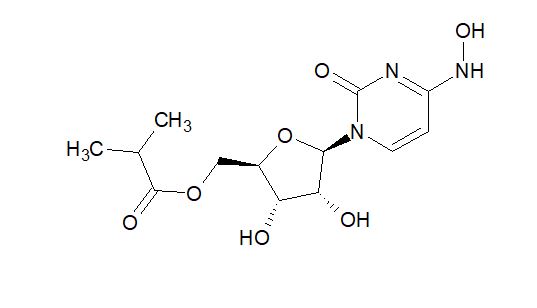 |
Block RNA polymerase | Suggested | Ridgeback Biotherapeutics Developed by Emory University |
| Activase | Tissue plasminogen activator Stroke drug |
Suggested | Roche (Genentech) |
| Actemra (tocilizumab) Monoclonal antibody |
anti-IL-6R Rheumatoid arthritis |
Approved in China 5 Clinical Studies in Denmark, Italy, China NCT04317092 NCT04331795 |
Roche, Medical institutions worldwide |
Umifenovir (Arbidol)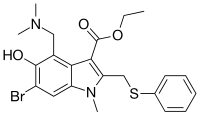 |
Membrane fusion inhibitor | Latest stages clinical studies in China (Including combinations with other drugs) ChiCTR2000029621 NCT04260594 |
Ruijin Hospital, Other institutions in China |
| 318H Monoclonal antibody |
Selectively targets cells having an abnormally high level of glycolysis and oxidative stress Aggressive cancers. |
Preclinical Development | SIWA Therapeutics |
| TAK-888 (Plasma-derived antibodies) |
Polyclonal hyperimmune globulin (H-IG) |
Development stage | Takeda |
Ruxolitinib (Jakafi, Jakavi)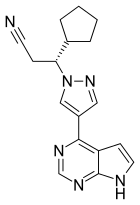 |
Inhibitor of Janus-associated kinases (JAK1 and JAK2) Myelofibrosis |
Clinical Study in China Ruxolitinib combined with stem cell therapy ChiCTR2000029580 Tongji Hospital, Hubei, China |
Tongji Hospital, Hubei, China Manufacturer - Incyte Corporation |
Camostat mesylate (Foypan)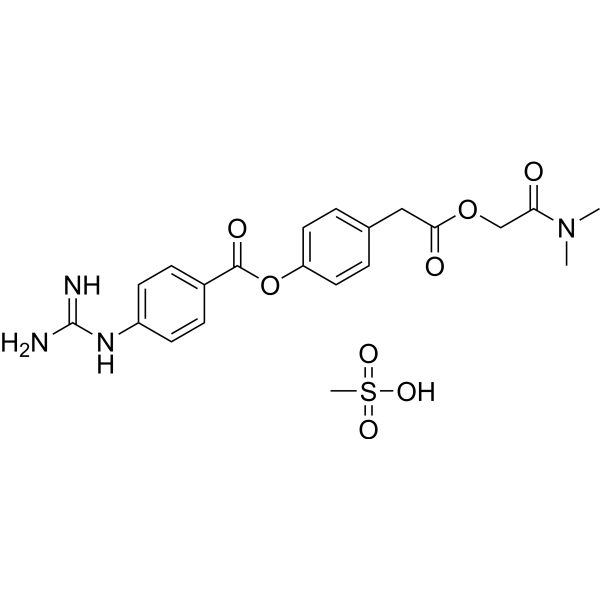 |
inhibit SARS-CoV-2 Spike protein-initiated membrane fusion | Phase 1, 2 Clinical Study in Germany NCT04321096 |
University of Aarhus Manufactured in Japan |
Nafamostat mesylate (Fusan) |
inhibit SARS-CoV-2 Spike protein-initiated membrane fusion Acute pancreatitis |
Completed preclinical study in Japan | University of Tokyo |
| Hydroxychloroquine (Plaquenil)  |
Endosomal acidification fusion inhibitor Anti-malarial Rheumatoid arthritis treatment |
> 10 Clinical Studies worldwide 10 Clinical Studies in China NCT04321278 NCT04261517 ChiCTR2000029868 |
|
| VIR-7831, VIR-7832 (sotrovimab) | Immunoglobulin Intravenous (Human) Monoclonal Antibody Directed Against SARS-CoV-2 | phase II/III NCT04779879 NCT04913675 | Vir Biotechnology in collaboration with GSK |
| COVID-HIGIV | Monoclonal Antibody Directed Against SARS-CoV-2, | Phase I NCT04661839 | Emergent BioSolutions |
|
MK-4482, EIDD-2801 |
Oral antiviral medicine | Phase III NCT04575597 |
Merck Sharp & Dohme Corp. |
Topics: Novel Therapeutics

Comments:
add Corbavax, Lactoferin, Hydroxichloroquin,
Leave a Reply cancel reply
Your email address will not be published. Required fields are marked *