Quarterbacking a Patient's Fight Against Cancer
In the incredibly competitive world of drug development, comprehensive precision, and healthtech, every strategic control point is essential. Over the next several months, during this editorial series, we will admittedly push the envelope as we elucidate several strategic control points in precision medicine, healthtech, drug development, clinical trial design, etc. We will weave in and out of these "trees" in a way few are capable of, at breakneck speed, as we elucidate the clinical "forest" comprised of them. As a full-time general hematologist/oncologist specializing in precision medicine, consultant to numerous pharmaceutical companies, CEO and Founder of two AI dependent companies in stealth mode, medical director of two phase 1/2 Xbiotech trials (pancreatic cancer), and having an MD/PhD (biochemistry)/MBA, I hope to offer you a unique perspective at the interface of AI, drug development, clinical trial design, healthtech, and precision medicine. In the last installment of this series, I described playing chess against cancer in the long-term approach to cancer patients. I discussed the refined integration of conventional therapy, precision medicine, and clinical trials via the COMET algorithm in the creation of patient-specific treatment maps, a term I coined treatment cartography. Today we reside in the moment and focus on the immediate care of cancer patients. To this end, whereas the long-term approach to cancer patients is a game of chess, the short-term approach is a game of American football.

Image credit: Adrienn Harto
Disclaimer:
- All patient images used in this editorial are completely deidentified. All patients whose images were used in this editorial consented to their use.
- Equating the long-term approach to cancer care to chess in the last editorial, and the short-term one to football here is solely being done to convey the thought process of the oncologist. In no way is it meant to trivialize what cancer patients experience. My patients know that they are part of my family at an exponential level. Please see a lecture I gave on youtube.com called "optimizing your chances at surviving cancer", or read my online patient reviews for confirmation.
American Football Primer
In American football two teams compete to score the most points by the end of four 15-minute quarters (two 30-minute halves). Teams can score in numerous ways, including scoring a touchdown by getting the ball into the part of the field called the endzone (figure 1).

Figure 1: Scoring a touchdown involves getting the ball into the endzone [1].
The two teams have eleven players each and alternate between playing offense and defense. The offense (figure 2) is comprised of the quarterback (QB), offensive line (LT, LG, C, RG, RT), and skill positions, including tight ends (TE), wide receivers (WR), running backs (HB), and full backs (FB). The goal of the offense is to score points, and the goal of the defense is to stop the offense.
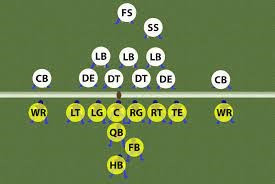
Figure 2: The offense (yellow) is comprised of the quarterback, offensive line, and skill positions. The defense (white) includes 11 players with designated positions.
A football field, upon which the game is played, is 100 yards and bounded by two endzones (figure 3).
Figure 3: A football field is 100 yards, bounded by two endzones [2].
The offense has four plays (downs) to gain ten yards, otherwise known as "moving the chains" (figure 4). If they are successful, they get an additional four downs to pick up the next ten yards, and so on and so forth. Failure to obtain ten yards in four plays results in the defense getting the ball. If a team scores on offense the roles are reversed as the defensive team goes on offense, and vice versa. The team with the highest number of points at the end of the game wins.
Figure 4: The offensive team has four plays (downs) to pick up 10 yards to move the orange chains [3].
In the short-term care of cancer patients, oncologists are the quarterback of the patient's offense pitted against the patient's cancer, the defense. The goal of the oncologist is to score as many points against the cancer as possible. Unfortunately, whereas the stakes of a traditional American football quarterback involve wins and losses, and an occasional trophy, the oncologist is often playing for the patient's life.
Game of inches
In the movie, Any Given Sunday, Al Pacino addresses his team at halftime of a playoff
game they are losing (figure 5):
"I don’t know what to say really. Three minutes to the biggest battle of our professional lives all comes down to today. Either, we heal as a team, or we are going to crumble. Inch by inch, play by play... You know, when you get old in life things get taken from you. I mean, that’s a part of life, but you only learn that when you start losing stuff. You find out life’s this game of inches, so is football. Because in either game, life or football, the margin for error is so small. I mean, one half a step too late or too early and you don’t quite make it. One half second too slow, too fast, and you don’t quite catch it. The inches we need are everywhere around us. They’re in every break of the game, every minute, every second. On this team we fight for that inch. On this team we tear ourselves and everyone else around us to pieces for that inch. We claw with our fingernails for that inch. Because we know when we add up all those inches that’s gonna make the freaking difference between WINNING and LOSING, between LIVING and DYING!"

Figure 5: Al Pacino, in "Any Given Sunday", addressing his team at halftime [4].
The Tennessee Titans were 36 inches away from scoring a game winning touchdown against the St. Louis Rams during Superbowl 34 (figure 6a). The Seattle Seahawks needed six inches to defeat the New England Patriots in Superbowl 49 (figure 6b). David Tyree's incredible "helmet catch" in Superbowl 42 that enabled the New York Giants to prevent the New England Patriots from being the best team in history was a "catch of inches" (figure 6c).

Figure 6: A game of inches for the Tennessee Titans (a)[5], Seattle Seahawks (b)[6], and New York Giants (c)[7].
Just like in football, the inches in medicine are literally everywhere. Correcting a patient's low sodium (hyponatremia), diabetic ketoacidosis, malignant hypertension, etc., too quickly can result in quadriplegia ("locked in syndrome"), hypoglycemic coma, or global ischemic brain injury, respectively. Conversely, addressing these issues too slowly can result in death. In hematology/medical oncology delaying antibiotic administration to a patient with a fever and absolute neutrophil count less than 500 for a mere hour can constitute the difference between living and dying. Failure to recognize autoimmune related side effects early when patients receive immunotherapy can result in irreversible damage and death. Failing to identify an actionable mutation in a patient's cancer can cost them years of their life. Accordingly, a constant awareness of the "inches" is critical for an oncologist to optimize their patient's chance at survival. To this end, being an oncologist has numerous parallels with being a National Football League (NFL) quarterback (figure 7).

Figure 7: Tom Brady, former quarterback of the New England Patriots, and current quarterback of the Tampa Bay Buccaneers [8].
The Offense
NFL offenses, as aforementioned, are often comprised of the offensive line, quarterback, wide receivers, tight ends, and running backs. The NFL quarterback calls the play in the huddle, walks to the line of scrimmage, where the football is placed, and executes the play. At the line of scrimmage, the quarterback reads the defense and reevaluates their offensive options. Their goal is to score the maximum number of points over the course of the game by maximizing the yardage they gain with each play.
At the line of scrimmage in the clinic, the oncologist stares down the patient's cancer. Like the NFL quarterback, the oncologist needs to select the correct play, understand the opponent, look for weaknesses in the defense, change the plan as warranted, and execute.
No quarterback can perform optimally if they don't have time to survey the field and make the right decision. The offensive line (figure 8) for oncologists, comprised of nurses, pharmacists, etc., clinic managers, etc., is essential to providing the guidance and assistance needed to optimally combat cancer. They are just as important members of a cancer patient's team as the oncologist.

Figure 8: The offensive line is an integral component of every team [9].
Just like an offensive line can impact winning and losing, great skill position players do as well. Where would Patrick Mahomes have been without tight end Trevis Kelce and wide receiver Tyreke Hill? Where would Tom Brady have been without tight end Rob Gronkowski? Where would Kurt Warner have been without wide receivers Torry Holt and Isaac Bruce, and running back Marshall Faulk?
In cancer care the quality of the treatment options available to you, the skill position players (receivers/running backs/tight ends), will absolutely make or break you. A tremendous wide receiver, such as osimertinib in EGFR exon 21 mutated metastatic non-small lung cancer, can allow you to gain huge yardage and move ahead of the cancer. The patient in figure 9 had complete regression of their brain metastases 13 days after starting osimertinib [10].
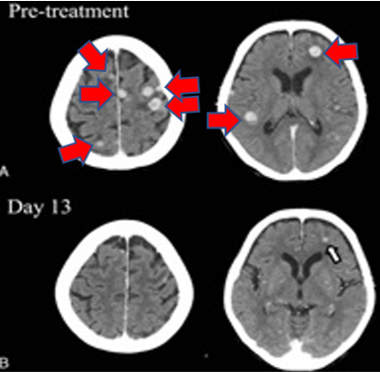
Figure 9: Osimertinib induced regression of brain metastases (red arrows) in only 13 days (top two scans are pre-treatment and bottom two are after).
Bad treatment options with poor risk-benefit ratios (e.g., regorafenib in stage 4 colon cancer) generally cost you yardage and doom you to failure. To this end, one is always looking for the best wide receivers, running backs, and tight ends via precision medicine and associated molecular profiling, clinical trials, etc., by employing the COMET algorithm presented in the first article of this series [11]. The mandate for pharmaceutical companies, drug developers, precision medicine corporations, etc., is to provide oncologists with the ideal skill players to best the defense and definitively win the game for patients.
The other team
In the NFL, like any sport, there are challenging teams and easier ones. Similarly, in cancer, sometimes the opponent is characteristically facile (e.g., classical Hodgkin lymphoma) and sometimes it's remarkably daunting (e.g., stage 4 anaplastic thyroid carcinoma). This often depends on the tumor type, molecular profile of the cancer, the "biology of the tumor", etc.
Collectively, we are very good at treating many early-stage cancers and numerous stage 4 malignancies, including Hodgkin Lymphoma, many B-cell non-Hodgkin lymphomas, testicular cancer, multiple myeloma, chronic myelogenous leukemia, etc. In contrast, we remain relatively poor at treating other stage 4 cancers including triple-negative breast, anaplastic thyroid, pancreatic, esophageal, gastric, head and neck, etc. Indeed, the very first mandate for the oncologist is to delineate who their opponent is.
It's imperative to recognize no two tumors are created equal, even if they have the same stage and histology. Cancer is not binary and always resides on a continuum from indolent to aggressive. Even among generally aggressive metastatic melanomas there are some that grow slowly and are more amenable to treatment than others.
Figures 10 and 11 depict two patients with BRAF V600E mutated metastatic melanoma at the time they presented to the oncologist. In figure 10, the patient had profound tumor burden involving the liver, bones, numerous lymph nodes, etc. [12]. Her malignancy is conspicuously aggressive and will undoubtedly kill her within months without treatment. In contrast, the patient in figure 11 has a solitary lung lesion and no additional tumor burden. Her tumor appears to be indolent and allows for a theoretically curative approach involving immunotherapy and surgical wedge resection of the lung lesion. Accordingly, the quarterback facing these two opponents has very different challenges, likelihoods of success, and treatment considerations.
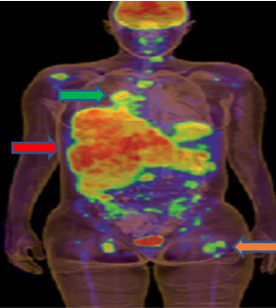
Figure 10: A patient with aggressive BRAF V600E mutated metastatic melanoma involving the liver (red arrow), bones (orange arrow), and lymph nodes (green arrow).
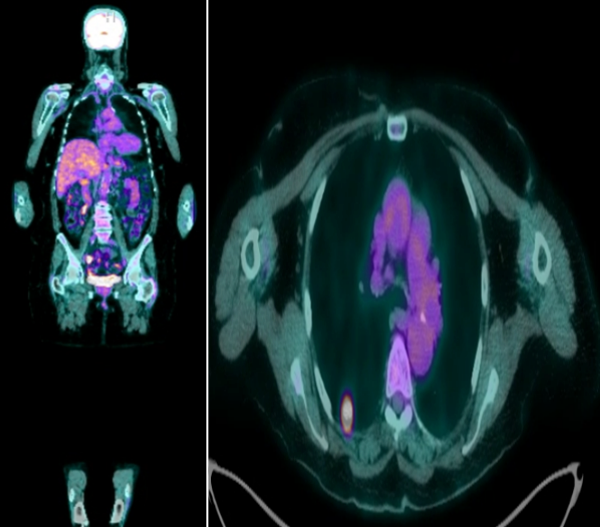
Figure 11: Patient with BRAF V600E mutated metastatic melanoma manifesting as a solitary right lung lesion.
There is little we comprehend about the heterogeneity of tumor biology from patient to patient, such as those in figures 10 and 11, and even in the same patient who has multiple metastatic sites of disease that respond differently to treatment.
The 80-year-old female, depicted in figure 12, with metastatic triple-negative breast cancer (12a) was treated with three cycles of carboplatin/gemcitabine (12b). The right axillary lymph nodes and right pelvic lesion responded beautifully to treatment, but two new liver lesions developed. This mixed response to treatment, representing underlying tumor heterogeneity, prompted a change in treatment to sacituzumab, and is the bane of a oncologist's existence.
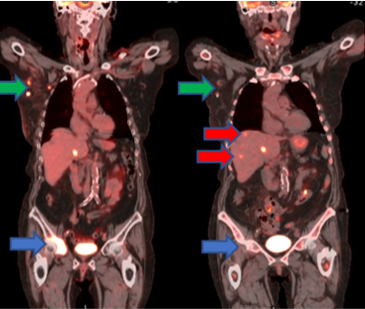
Figure 12: A patient with triple-negative breast cancer before (a) and after (b) three cycles of carboplatin/gemcitabine. The right axillary lymph nodes (green) and right pelvic lesion (blue) improved, but two new liver lesions (red) developed.
The best oncologists are adept at ascertaining the strength of their opponent by delineating the underlying tumor biology. This is derived from observing the tumor's initial tumor burden, growth rate, response to treatment, molecular profile, histology, sites of metastases, etc. With every restaging scan performed after a designated treatment the oncologist learns more about the opponent and adjusts accordingly.
Consider the patient illustrated in figure 13 with metastatic, HER2-overexpressing, PD-L1 positive, metastatic esophageal cancer manifesting as a left malignant pleural effusion, large esophageal mass that precluded them from eating, and diffuse lymph node involvement (figure 13, panel A). The patient was initiated on FOLFOX (5FU, leucovorin, oxaliplatin), keytruda (pembrolizumab) due to PD-L1 positivity of the tumor, and herceptin (trastuzumab) to target HER2. After four cycles the patient was in a complete remission, and remained so after cycle 8 of treatment (figure 13, panel B). At that time, he had substantial oxaliplatin-induced neuropathy. Accordingly, as I typically go to "maintenance therapy" after cycle 8 of FOLFOX anyways, and the patient was in a complete remission, I discontinued his oxaliplatin. He received four cycles of maintenance 5FU, keytruda, and herceptin, and remained in a complete remission (figure 13, panel C). Therefore, in an effort to omit chemotherapy entirely from his maintenance regimen, I stopped his 5FU. After four cycles of keytruda and herceptin the patient had profound difficulty swallowing as PET CT revealed his esophageal tumor worsened and his cancer progressed in several lymph nodes (figure 13, panel D).
Every scan I performed on the patient taught me more about his specific cancer's biology. Through the course of 8 months of therapy I learned that his tumor was very responsive to chemotherapy, but doesn't appear to be particularly sensitive to HER2 targeted therapy or immunotherapy. Importantly, as I'm always extremely careful to change one variable at a time, if possible, I demonstrated his tumor progressed only after all chemotherapy (5FU and oxaliplatin) was removed. Consequently, the two primary treatment options available to us after completion of the patient's last therapy, aside from palliatively radiating his primary esophageal lesion, was going back to FOLFOX and continuing herceptin to target HER2, or changing his treatment to Enhertu, approved for HER2-positive metastatic esophageal cancer in 2021. Ultimately, I elected to revert back to FOLFOX and herceptin and reserve Enhertu as a future play.
This patient's cancer has become a very challenging opponent as it progressed in merely three months on keytruda and herceptin, after chemotherapy was omitted. Nonetheless, this discussion demonstrates how oncologists are constantly accruing information pertaining to their opponent through patient symptoms, restaging scans, etc.
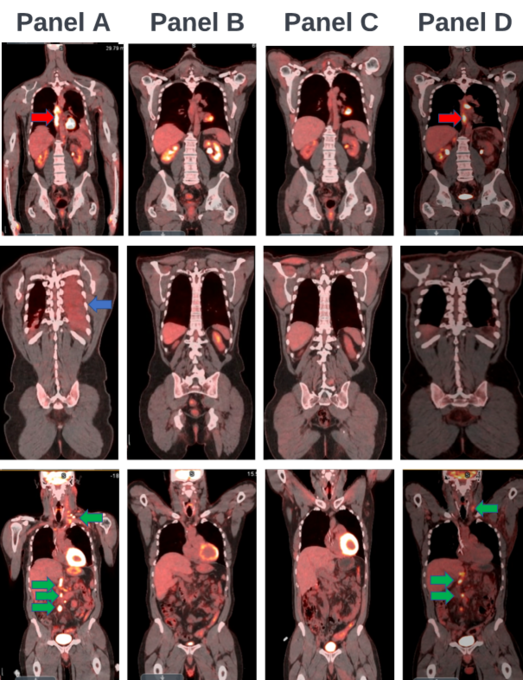
Figure 13: A patient with HER2+, PD-L1+, stage 4 esophageal cancer before (a) and after eight cycles of FOLFOX/keytruda/herceptin (b), four subsequent cycles of 5FU/LV/keytruda/herceptin (c), and four cycles of herceptin/keytruda alone (d). The right pleural effusion (blue arrows), lymph node metastases (green arrows), and primary esophageal tumor (red arrows) are labeled. The remaining PET CT uptake is physiologic and not malignant.
A sophisticated understanding of the opponent has a profound impact on the plays the oncologist calls and executes during the game. A challenging opponent, cancer with substantial tumor burden at onset, sometimes requires a completely different game plan than an easy opponent, a cancer with minimal tumor burden. The same holds true for a cancer that rapidly progresses through treatment versus one that enters a complete remission. For example, although we initiate treatment on nearly all stage 4 lung cancers at onset there are occasional patients whose tumors grow so slowly some may monitor them off treatment. CLL is a disease we routinely take a watchful waiting approach with, but some patients, like the one in figure 14, have extensive disease that requires immediate therapy; this patient was started on acalabrutinib/rituximab with marked reduction in her large abdominal lymph node conglomerate.
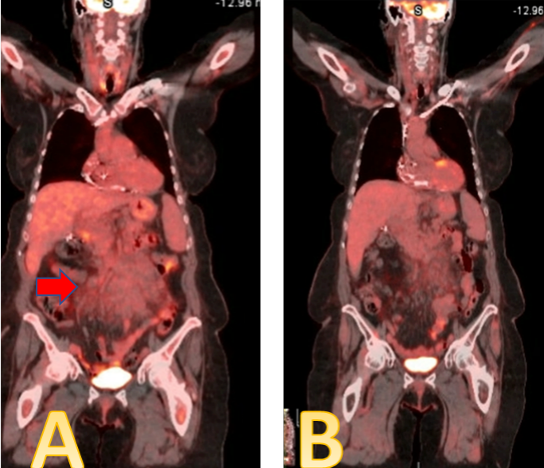
Figure 14: A patient with CLL manifesting as a large intrabdominal lymph node conglomerate (A-red arrow), markedly reduced after three months of acalabrutinib/rituximab (B)
There are 1,093,234 high school football players in the United States. 71,060 (6.5%) will play in college. Only 1.2% of those will play in the National Football League [13]. Accordingly, even winless NFL teams have very good players that are capable of winning when least expected. Similarly, even "easy" cancers, such as Burkitt's lymphoma, chronic myeloid leukemia, testicular cancer, and classical Hodgkin lymphoma, that are essentially cured 80-90% plus of the time, can defeat you. An oncologist can never take their opponent for granted, and must remain forever vigilant.
The Playbook
In football, teams compile the list of their offensive and defensive plays in a playbook (figure 15).
Figure 15: Example of plays in an NFL playbook [14]
Quarterbacks select a play from the offensive playbook and then execute it. Similarly, once the oncologist has developed an understanding of the patient's cancer, they select a play from their treatment playbook. In cancer care, the type of playbook you use, corresponding to all available therapeutic interventions for your patient, is entirely dependent on tumor type. Although precision medicine is allowing the same play to be used in multiple playbooks, cancer treatment is still very much tumor and patient dependent. The playbook is relatively small for a patient with stage 4 rhabdomyosarcoma, and is sizeable for one with multiple myeloma. If the patient is wheelchair bound and can't tolerate treatment sometimes the only play in the playbook one can use is enrollment in hospice. In contrast, if the patient is fully functional one can potentially access every play in the playbook. Later in this editorial series we will describe how precision medicine, AI, and bespoke drug development are leading us closer to the day every patient may have their own personalized playbook.
Down and distance
In football you have four downs (plays) to move the football ten yards. Accordingly, the down and distance play an instrumental role in which play you call. In the care of patients, a first and 10 situation corresponds to an oncologist having numerous plays they can call while knowing they have additional chances (downs) to contain the cancer. If it's second and 1, as in the case of a patient with minimal tumor burden, the oncologist can afford to take an approach with higher upside; a more durable treatment response. If it's fourth and 30, such as when a patient has widely metastatic disease compromising organ function, there is no time to lose as the oncologist has limited options and one play to save the patient's life.
Consider a 45-year-old female patient with stage 4, BRAF V600E mutated, melanoma. Global options for this patient include targeting the BRAF V600E mutation with a BRAF V600E inhibitor/MEK inhibitor combination (dabrafenib/trametinib, vemurafenib/cobimetinib, or encorafenib/binimetinib), or immunotherapy (opdualag, ipilimumab+nivolumab, nivolumab, pembrolizumab). Theoretically, one could use both strategies upfront with vemurafenib/cobimetinib and immunotherapy (atezolizumab), but recent data suggests this isn't optimal [15]. Regardless, for our purposes we will assume we have two predominant options outlined above.
BRAF V600E/MEK inhibitors have remarkable efficacy in BRAF V600E stage 4 melanoma with response rates of approximately 75-80% and median duration of response of 12 months. Moreover, these drugs are miraculous as they can induce marked tumor regression in weeks. In contrast, immunotherapy has response rates of approximately 40-50% in BRAF V600E mutated tumors and takes longer to work. However, it is critical to recognize in the clinic, and for our purposes, that immunotherapy, unlike BRAF V600E/MEK inhibitor combinations, can induce very long-term remissions essentially equal to cure. As such, although the BRAF V600E/MEK inhibitor combination has a higher chance of working and works faster, immunotherapy has a decent response rate and can work much longer.
With this in mind let's consider a 45-year-old, stage 4, BRAF V600E mutated melanoma patient (figure 16). If at the time we meet her we find it's second and 1 due to her having very minimal metastatic disease, such as in figure 16a, we could use either the BRAF V600E/MEK inhibitor combination or immunotherapy. Using the BRAF V600E/MEK inhibitor would be equivalent to a pass play that you are nearly certain will get you the first down and/or touchdown, but will not definitively win the game via a theoretical cure. Using immunotherapy would be equal to throwing a deep crossing route with a reasonable chance of success that could yield a gigantic gain, touchdown, or definitively win the game. You would not be wrong choosing either of these options, but I would use immunotherapy (ipilimumab+nivolumab) in this patient to chase the theoretical cure. I would do this knowing that I still had third and fourth down to pick up the first down, and could use my BRAF V600E/MEK play to safely move the chains if immunotherapy failed.
Let's take our same 45-yo stage 4, BRAF V600E mutated melanoma patient and assume at the time we meet her it's fourth and 30, as in figure 16b, as she has diffuse metastases and profound liver involvement. Without treatment it's conceivable she would live less than one month without treatment. In this instance we have no time to wait for immunotherapy to work and we absolutely can't afford to be wrong while reserving our most effective option; it's already fourth down. Thus, we would choose the safer play and begin the patient immediately on a BRAF V600E/MEK inhibitor combination (e.g. dabrafenib/trametinib).
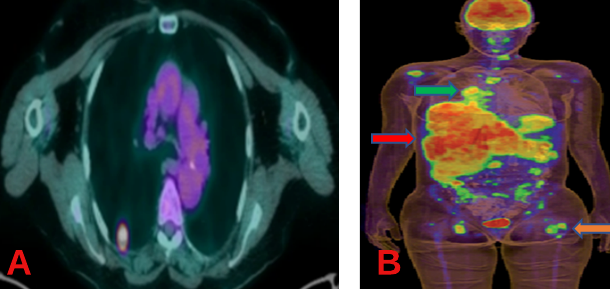
Figure 16: Two patients with metastatic, BRAF V600E mutated, melanoma, but with completely different down and distance situations: second and 1 (A), and fourth and 30 (B).
Figure 17 demonstrates two patients with widely metastatic BRAF V600E mutated melanoma before and after only two weeks of vemurafenib, a BRAF V600E inhibitor [12]. Our patient from figures 10 and16b, depicted as patient 1 in Figure 17a, who was on the absolute brink of death, miraculously is in a complete remission after only two weeks of vemurafenib; a fairly well tolerated, non-chemotherapy, targeted BRAF V600E therapy. This is the equivalent to throwing a deep "go route" to a triple-covered wide receiver (BRAF V600E/MEK inhibitors) on 4th and 30, down 7 with no time left on the clock, and scoring a touchdown to get the game to overtime.
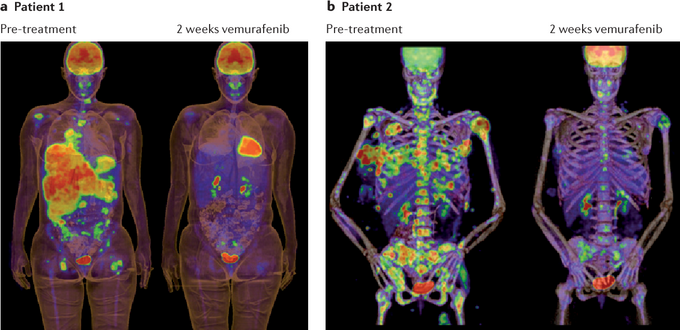
Figure 17: Two patients with metastatic, BRAF V600E mutated, melanoma, before and after being treated with only two weeks of the BRAF V600E inhibitor, vemurafenib. Patient 1, depicted earlier in figures 10 and 16b, is in a complete remission (the uptake noted on the after treatment scan is physiologic). Patient 2 is nearly in a complete remission.
It's warranted at this juncture to take a moment to truly appreciate the impact of the BRAF V600 mutation in patient 1's tumor and how incredibly critical genomic sequencing is in treating cancer patients. In hospitals throughout the country individuals like patient 1 are being reflexively enrolled in hospice and committed to death by physicians who KNOW LITTLE ABOUT CANCER. Indeed, it's very easy to envision the patient 1s of the world never having a chance to be treated with vemurafenib. This would have likely cost patient 1 at least a year of quality life as median duration of response, the median time before her tumor progresses, is approximately one year.
In medicine we have a morbid expression, "the patient isn't dead until they're warm and dead". We recognize that patients can survive profound hypothermia and it can actually be incredibly beneficial in patients with acute cardiac events. To this end, when I gave a presentation on precision oncology for Grand Rounds at the University of Buffalo in March of 2020, I titled my talk, "Cancer patients don't have an expiration date". I specifically stated that "a cancer patient isn't dead until they have been sequenced and dead". Although admittedly relatively rare in certain cancers. when patients are found to have actionable mutations in various genes, including EGFR, ROS1, ALK, NTRK, BRAF, etc., it can have a revolutionary impact on their prognosis and associated quality of life. In this editorial series we place tremendous emphasis on precision medicine, and encourage the reader to remain cognizant of its transformative nature.
The discussion of our 45-year-old stage 4 BRAF V600E mutated, metastatic melanoma patient is far more nuanced than described here, but the aforementioned thought process plays out daily in the clinic. The oncologist must always be aware of the down and distance as it can impact the play they call and execute.
So far, we have only talked about an oncologist gaining yardage, but with every play there is always a real chance they could LOSE yardage.In our second and one patient with BRAF mutated metastatic melanoma with a solitary lung lesion (figure 11), that we elected to use immunotherapy for, it's possible her cancer could progress on this, or that she could have side effects to the drug.Accordingly, rather than be faced with third down and 1 (figure 18a) if immunotherapy simply didn't work, we could be faced with third and ten (figure 18b), or worse, because the patient's cancer or their condition worsened.

Figure 18: The stark difference between 2nd and 1 (left) and 3rd and 10 (right). The yellow line in the right picture is the "line to gain" for the first down.
Consider the 60-year-old patient with stage 4, PD-L1 95% positive, EML4-ALK fusion positive, lung adenocarcinoma depicted in figure 18. At diagnosis the patient had numerous enlarged cervical, axillary, and mediastinal lymph nodes, and a central left lung primary tumor (19, panel A). The oncologist, faced with first and 10 at their own 20-yard line, down 7-0 to the patient's cancer, had two primary options. They could take advantage of the ALK fusion in the patient's tumor and throw the ball to their wide receiver, an ALK inhibitor, or they could throw to another receiver, conventional chemotherapy in the form of carboplatin/pemetrexed/pembrolizumab. The ALK inhibitor is associated with a higher response rate, less toxicity, and longer duration of response than chemotherapy. As such, it represents Jerry Rice, the best wide receiver in American football history, relative to Steve Largent, a former Seattle Seahawks wide receiver who was very reliable and one of my favorites of all time, but not as good as Jerry Rice. Throwing to Jerry Rice in this scenario is obvious, and the patient was started on brigatinib, an ALK inhibitor.
Three months after starting brigatinib the patient's lymph nodes and lung lesions resolved entirely and he was in a complete remission (figure 19, panel B). This is equivalent to throwing a bomb to Jerry Rice on first and 10 that results in a touchdown that ties the game.
The patient was monitored with imaging every three months, and had no evidence of cancer on scans for the next 13 months. Basically, as the defense had no answer, the oncologist simply called the same play over and over again. Thus, 17 months after first calling on brigatinib the oncologist's team is up several touchdowns on the cancer. Sadly, ALK inhibitors do not cure stage 4 lung adenocarcinoma as the defense essentially always adjusts. This manifest as progressive disease in a left hilar lymph node and left pleura, as the defense finally figured out the play being run on first down (figure 19, panel C).
At the time of progression, the oncologist now faces a second down for the first time in 18 months. At this point the oncologist can either throw to a similar receiver, an ALK inhibitor called lorlatinib that has been shown to overcome resistance to brigatinib, or to use the aforementioned chemotherapy. Unlike brigatinib earlier in the game, lorlatinib is not nearly as good a wide receiver at this juncture in the patient's care. Accordingly, the choice of what receiver to utilize, lorlatinib or chemotherapy, doesn't have a definitive answer. The decision resides on whether the oncologist is facing 2nd and 1 or 2nd and 10, which is entirely dependent on the patient's cancer burden. Due to the minimal cancer evident on the patient's PET CT the oncologist appears to be faced with a 2nd and 1 and has room for error. Specifically, the oncologist has 3rd and 4th downs to pick up any yardage they require. Accordingly, as lorlatinib has the least potential side effects, the oncologist selects lorlatinib.
Scans performed three months later show a new left pleural effusion, significant increase in the pleural disease, and new bone lesions (figure 19, panel D). This suggests that the oncologist actually LOST yardage on the 2nd and 1 play and is now faced with 3rd and 10. The only viable play now is to use conventional chemotherapy.
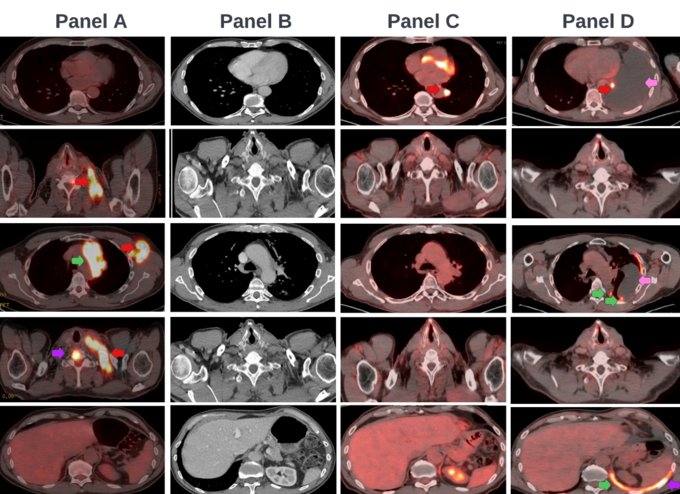
Figure 19: A patient with ALK fusion +, PD-L1+, stage 4 lung adenocarcinoma at diagnosis (a), after three months of brigatinib (b), at initial progression 18 months after starting brigatinib (c), and following three months of lorlatinib (d). The patient presented with tumor involvement of the left lung and pleura (green arrows), and lymph nodes (red arrows). He subsequently developed a left pleural effusion (blue arrows) and bone lesions as well (purple arrows).
Ultimately, as demonstrated above, very few things influence an oncologist's play calling as much as down and distance. The best oncologist assesses tumor burden, past behavior, etc., to accurately determine the down and distance, and best play to call.
Calling an audible
In the NFL quarterbacks frequently audible (change the play) at the line of scrimmage when they see how the defense is aligned (figure 20).

Figure 20: Tom Brady calling an audible at the line of scrimmage [16].
Similarly, in cancer care there are many times when you have a treatment (play) you expect to give a patient that you have to change in real-time based on a change in kidney function, blood counts, symptoms, tumor status, etc. Accordingly, oncologists must be phenomenal at calling audibles when needed for their patients as failure to do so can result in profound loss of yardage manifesting in a patient's death.
Frequent reasons to audible include progressive disease on restaging scans, chemotherapy induced neuropathy (taxol, taxotere, and oxaliplatin), cisplatin related nephrotoxicity and audiotoxicity, etc. Intrinsic to cancer care is idiosyncrasy, as patients may respond and tolerate treatment drastically different from one another. An oncologist simply can't take anything for granted in cancer care and must account for every inch, sometimes through calling an audible when least expected.
In our ALK fusion positive lung adenocarcinoma patient an audible was called the moment his scans revealed his tumor progressed on brigatinib. This manifest as a request for a biopsy of a new site of disease to ensure the tumor didn't change morphology, molecular profile, etc. The biopsy showed it did not and the play, brigatinib, was changed to lorlatinib.
Time and score
Good NFL teams understand the importance of time and score on play calling and execution. Although many early-stage tumors are highly curable and we often win against our opponent, most stage 4 oncologic tumors are incurable. As such, we ultimately end up losing more games than we win in this setting.
Every time an oncologist takes the field they start out behind. The cancer has already scored before you even touch the ball. Regardless, even though we lose most games when quarterbacking against stage 4 tumors, we have become exponentially better at extending the game over the last several decades. Presently, there are 20 million Americans living with cancer because of profound advances made in the last 10-20 years. Moreover, clinical trials provide hope where there was none. There are absolutely people alive today because they enrolled in a clinical trial years ago. Accordingly, sometimes as quarterbacks we are trying to extend the game through a clinical trial, novel therapeutic, etc. The hope is if we can extend the game long enough a new agent will present itself that offers the patient the chance at definitively winning the game.
At the beginning of the game the patient's performance status is often the best it will be, and is usually decent. We generally have time to be able to run numerous plays to try and score points and get ahead of the cancer. Even if the cancer is ahead, we have time to catch up. However, as the game nears the end, patients often have limited treatment options as the cancer has learned our playbook and is resistant to it. Usually by this time the cancer is so far ahead on the scoreboard there are no obvious plays we can run to get the game to overtime and extend it. In these situations, the only option is to let the clock run out (enroll in hospice), or throw a Hail Mary in the form of a long shot clinical trial; treatment option usually associated with a minimal response, a completely "outside the box" approach, etc. Unfortunately, aside from having less plays the cancer hasn't seen as the game progresses, patients have often been "tackled" so many times they have minimal energy as the clock nears expiring, thereby limiting the plays one can run.
At every step in the game decisions are made by the oncologist and patient in lockstep, as though the patient was talking to the oncologist via a headset. To this end, I always tell my patients:
"I'm the navigator, but you're the captain. I lay out the potential courses we can take, but it's your boat, you decide which route we're taking. It's my job to get you to whatever destination you choose safely."
The patient really is the one affirming every play call and the oncologist's job is to execute it. As such, I always tell patients that it's ultimately their decision if the "juice is worth the squeeze" (figure 21).

Figure 21: "Is the juice worth the squeeze?" conversation underlies every facet of cancer care [17].
I spend excessive amounts of time educating the patient and their family so they fully understand the squeeze/juice ratio. Empathy plays a paramount role in this as patients may perceive the juice and squeeze differently than you and other patients, even if they have similar tumor types and stages. Much of what we will discuss in future articles in this series is directed at optimizing the squeeze/juice ratio of our treatment options
Trick plays
NFL teams, although they generally run conventional plays, sometimes use innovative trick plays to win games. The best hematologist/medical oncologists are no different. They are lucky to have tremendous resources, including NCCN guidelines, to provide a reference guide. It's akin to having access to a cookbook for cancer care as these guidelines are generally based on the thoughts of key opinion leaders. Generally, if oncologists are deviating from them there should be reasonable justification. However, as the medical world has rightfully become more evidence-based, we are witnessing the death of intuition and creativity in medicine, particularly in hematology/medical oncology. We forget that NCCN guidelines are "guidelines", not dogma. We discuss phase 1 trial and phase 2 trial data as though it was definitive. Yet, some estimate the number of phase 1 drugs that are ultimately FDA approved to be approximately 8-14%. Moreover, studies have shown that only about 30% of phase 2 trials are positive, and only 47% of oncology drugs with successful phase 2 trials are positive in phase 3 [18-19]. In addition, many patients present in a way that no trial or NCCN guideline can possibly account for, largely due to nuances in cancer biology and presentation.
Cancers don't read a book. They don't care about what our prognostic assays predict will happen. They are unaware of past and present clinical trial data. There is no set of guidelines that can encapsulate every possible scenario hematologists/oncologists face in the clinic. Accordingly, there remains a marked role for measured creativity and intuition in the treatment of hematology/medical oncology patients, in the appropriate settings. This sometimes manifests in trick plays, outside the box approaches hematologists/oncologists run to fight cancer, and is a key differentiator between good and great physicians. This can entail extrapolating therapies from one tumor to another if data to the contrary is lacking, insurance allows it, and the squeeze/juice ratio is auspicious. In fact, this is often the inspiration for novel clinical trials and drug development endeavors.
Run Versus Pass Play
In football there are plays that allow the quarterback to hand off to the running back or throw to a receiver. Generally, the run play has the lowest risk, but also is less likely to pick up the biggest gain. In contrast, pass plays generally have the highest risk and upside.
Deciding whether to choose a run or pass play as a quarterback of a patient's cancer care, as already discussed, depends on time and score, down and distance, etc. Moreover, the talent of your skill positions, running back, wide receivers, etc. influences this decision. Depending on the play call there is a risk of the running back fumbling or the pass being intercepted. These risks are akin to side effects patients may encounter with the treatment option you choose. Some drugs will have higher risk, or different risks, than others. Deciding on whether or not to hand off to a running back or throw to a receiver always involves a squeeze/juice ratio assessment. There may be a receiver running a twenty yard out route that you are certain can be completed with minimal risk of an interception. This is akin to a play with a favorable squeeze/juice ratio, high success and low side effect rates, such as gleevac in the treatment of CML. Alternatively, you may elect to throw a bomb to a double-covered receiver that has a high chance of being intercepted, but if completed nets considerable yardage. This would be akin to a high squeeze/high juice treatment like 7+3+midostaurine induction followed by autologous hematopoietic stem cell transplant for AML with a FLT3 ITD mutation and complex cytogenetics. Ultimately, making the appropriate play call and executing the play properly can be the difference between a patient living or dying.
Throwing to a wide open receiver
In football, poor defense can result in a wide receiver being wide open (figure 22). Often times we perseverate on minutia in medicine. We feel we have to hit the "medical target" perfectly, that there is only one right way to treat a patient. As physicians become more skilled, they often recognize that is generally not the case. Often times the question has more than one right answer, and the therapeutic target isn't as small as one would think. Sometimes the receiver really is wide open and the oncologist doesn't have to throw a perfect pass for it to be caught. The use of lorlatinib, brigatinib, ceritinib, or alectinib are examples of this as several ALK inhibitors can be used due to their excellent squeeze/juice ratio. Knowing when this is the case requires mastery of your respective field. Assimilating this with the notion that the inches do matter in many situations in medicine absolutely separates the best oncologists from their colleagues.
Figure 22: Throwing the ball to a wide open receiver [20].
Timeouts
In NFL games teams call timeouts to stop the clock in an effort to extend the game. In addition, it provides players with time to rest. In cancer care there are many times one wishes they could just stop the game. However, this isn't possible. Yet, the way to call timeouts as an oncologist is to give patients a treatment break to allow them to recuperate and fight another day. In fact, the patients with stage 4 metastatic pancreatic cancer that live for inordinate long periods of time generally don't do so because their oncologist is better than everyone else. Indeed, it likely has very little to do with the oncologist and more with the tumor biology; some stage 4 pancreatic cancers are more indolent than others. Yet, a way oncologists can potentially maximize a patient's longevity is recognizing when they can call timeout and "safely" give the patient a treatment hiatus. To this end, if a patient has minimal tumor burden and their disease is well controlled, I will sometimes stop the patient's chemotherapy and do a short-interval restaging CT scan in two months. If the disease remains stable, I will continue to hold treatment and restage two months later. This allows the patient to remain off treatment and avoid the considerable side effects associated with the only available treatment regimens: FOLFIRINOX, 5FU/LV/onivyde, and gemcitabine/abraxane. In fact, I consider time off treatment the "Holy Grail" in many malignancies, especially stage 4 pancreatic cancer, but this requires considerable understanding of the opponent (tumor biology and burden, heterogeneity, etc.). Unfortunately, despite being able to call a timeout at times, the game always resumes as cancer almost never forfeits.
Defensive Adjustments
In the NFL the defense is always adjusting to what the offense is doing (figure 23).
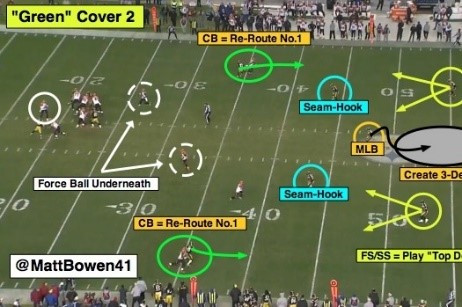
Figure 23: Defensive coverages and adjustments in football [21].
Similarly, cancer is never static. Resistance to treatment can develop via mutations, changes in the microenvironment, protein expression profiles, etc. Accordingly, even though we can often successfully eradicate large swaths of stage 4 tumors, many of them remain incurable. The oncologist is constantly having to change the play call based on the defense making unexpected adjustments.
Consider the 85-year-old patient in figure 24 with stage 4AE, germinal center, non-double hit, diffuse large B-cell lymphoma (DLBCL). The first time the oncologist takes the field they are behind on the score as the patient has cancer involving the liver, spleen, bone, and lymph nodes (figure 24, panel A). Initial assessment of the opponent reveals an aggressive malignancy, but one where there is a greater than 60% chance of winning the game. The initial play call is obvious, and the patient is started on RCHOP (rituxan, cytoxan, adriamycin, vincristine, and prednisone), with a planned six cycles of treatment. 9 weeks later, after the patient received 3 cycles of RCHOP, PET CT revealed regression of the patient's liver, bone, and spleen lesions (figure 24, panel B). However, two small lymph nodes remained evident (figured 24, panel B). Regardless, treatment was successful and the patient pulls ahead of the cancer. Without changing the play call the patient receives three more cycles of RCHOP. Surprisingly, subsequent PET CT revealed marked progression of two lymph nodes, as the cancer changed the defensive coverage and figured out the RCHOP play. Accordingly, whereas the oncologist had called a play, simply monitoring the patient, feeling the patient's tumor would be cured after six cycles of RCHOP, the oncologist now has to call an audible due to unexpected tumor progression. Due to the mixed response to treatment, the oncologist requested a biopsy of the para-esophageal lymph node that demonstrated persistent diffuse large B-cell lymphoma.
At this time the oncologist can select from numerous plays. However, it's important to note the playbook available to the oncologist in this 85-year-old patient is different than if the patient were younger. It doesn't include CAR-T cellular therapy, such as yescarta, or high-dose chemotherapy with a salvage autologous hematopoietic stem cell transplant (bone marrow transplant). Regardless, the playbook does include several viable options as the patient's tumor makes CD19, including:
- Polatuzumab+bendamustine+rituxan (pola+BR)
- Tafasitamab+lenalidomide
- Loncastuximab
Each of these options has distinct squeeze/juice ratios, encompassing their potential side effect profile, chance and magnitude of success, patient time and financial toxicity, etc. Ultimately, the pola+BR play is called. After three cycles of treatment, the patient's disease progressed (figure 24, part D) and the cancer has pulled ahead on the scoreboard. Moreover, as we consider time and score, the game is disconcertingly near the end. The patient is exhausted as the squeeze/juice ratio is very different at this juncture than it was when treatment started. As such, the patient strongly considered enrolling in hospice, but was amenable to one last play, tafasitimab plus lenalidomide.
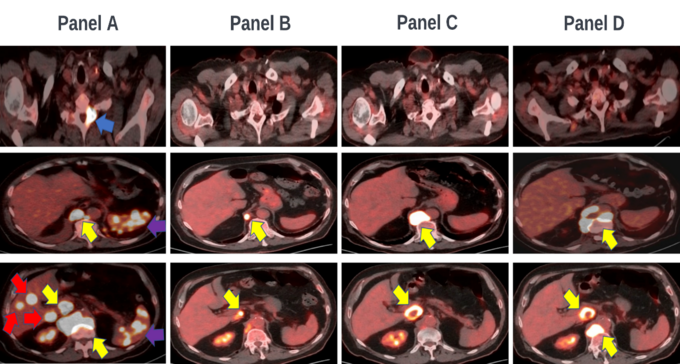
Figure 24: 85-year-old male with DLBCL (a) after 3 cycles of RCHOP (b), 3 additional cycles of RCHOP (c), and three cycles of pola+BR(d). Lesions in the lymph nodes (yellow arrows), liver (red arrows), bone (blue arrow), and spleen (purple) are marked.
Penalties
In football, teams incur penalties, represented by yellow flags, of varying severity when they commit infractions (figure 25).

Figure 25: Referee throwing a flag while calling a penalty [22].
In cancer care patient side effects constitute penalties that can cost considerable yardage and result in death. No treatment, regardless of how little juice it produces, doesn't require a squeeze; every therapy has an associated risk. Accordingly, oncologists are constantly weighing the squeeze/juice ratio; potential side effects versus putative efficacy. The squeeze/juice ratio differs among therapeutics and is dependent on the intervention type, mechanism of action, etc. In the patient with metastatic EGFR exon 21 L858R mutated, PD-L1 negative, MSI-negative, and TMB-negative, metastatic non-small lung cancer, the squeeze/juice ratio of osimertinib is remarkably favorable and far better than it is for chemotherapy (carboplatin, pemetrexed, and pembrolizumab). As such, standard first-line therapy in such a patient is osimertinib, and is typically associated with a low risk of penalization. However, in a patient with metastatic leiomyosarcoma with no favorable molecular markers the squeeze/juice ratio for every available therapeutic is unfavorable. One can consider using adriamycin with ifosfamide, a regimen capable of producing the most juice among conventional treatment options, but also the highest risk of penalty (squeeze). Alternatively, one can use trabectedin, a drug with a much lower squeeze (risk of penalty) but also a much lower production of juice (progression free survival). Choosing adriamcyin plus ifosfamide in this scenario may allow for a considerable gain of yardage that picks up the first down, but could also result in a massive penalty with a profound loss of yardage leaving the oncologist and patient with a third and 30, as opposed to a first and 10. This would limit the use of any other additional treatment options capable of picking up the first down, and could hasten a patient's demise. Accordingly, when deciding on what play to call and execute oncologists must consider comparative squeeze/juice ratios and associated risk of penalization.
Final Analysis
Over the last 11 years as a general hematologist/oncologist I have played for numerous patients and confronted many opponents. I have used countless playbooks, many of which have changed dramatically over the last decade. I've never been as hopeful as I am today that we will see an explosion of quality skill players in the future, and that games we used to have no chance at winning will become winnable. I encourage the reader to consider all the playbooks we use to combat various opponents to identify profound areas of unmet need. The continued evolution of precision medicine will reveal several plays in the future that can be extrapolated form one playbook to another.
As a hematologist/oncologist who gives patients his cell phone number and treats every patient like family, I am nervous every time I open a patient's scan to see if the play called was successful or not. I honestly feel visceral anger when a patient's tumor progresses or it feels like the cancer is cheating; scans show a mixed response to therapy or a patient presents with multiple cancers at once. With that said there are few joys greater in life than to show a patient their scans when they are clearly "winning" (figure 26).
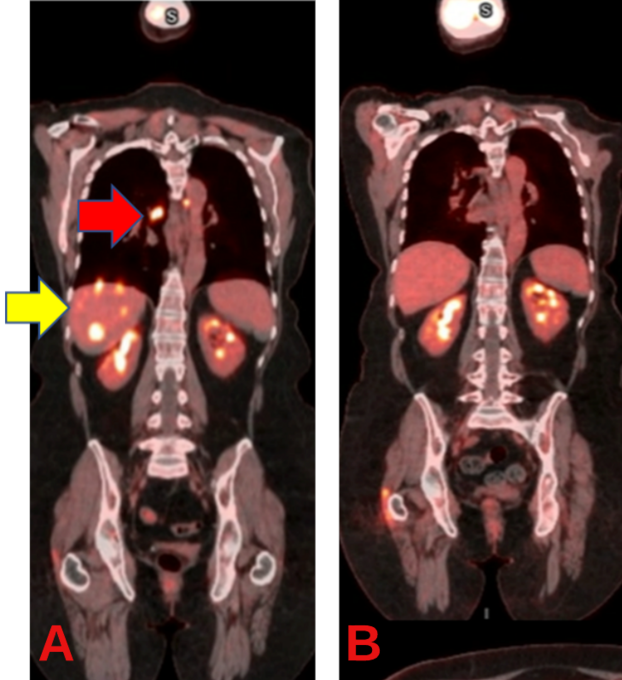
Figure 26: Patient with estrogen receptor positive, progesterone receptor positive, HER2-positive, breast cancer before (A) and after (B) TCHP therapy revealed a complete remission. Liver (yellow arrows) and lymph node (red arrows) lesions are noted. The uptake in the kidneys is physiologic, and not malignant.
Ultimately, like NFL quarterbacks, an oncologist must know their opponent, the playbook, the players, time and score, down and distance, risk of penalty, when to call timeout, when to audible, and when a receiver is wide open. At the heart of this they must remember cancer care, like football, is a game of inches. They can't take anything for granted and must always question when the defense does something unexpected. They must be prepared to ask the necessary "why?" questions? Why is the cancer showing a mixed response? Why is the cancer progressing on treatment? Why does the patient have these side effects? Indeed, not just in cancer care, but in life, the "why?" questions are critical to success.
In the last installment of this series, I described playing chess against cancer in the long-term approach to cancer patients. I discussed using the COMET algorithm during patient specific treatment cartography. Here we focused on the short-term approach to cancer care through the lens of American football. In so doing, we provided the reader with a never before seen look at the mind of an oncologist. This was done because everyone fighting cancer, whether it be pharmaceutical, healthcare, healthtech, or precision medicine employees, can benefit from a fundamental understanding of the clinical approach to cancer. With this in hand, the next entries in this series, previewed below, will embark on an unprecedented tour of bona fide translational medicine that I hope you will join me for.
The Insider's Guide to Translational Medicine
This is an unprecedented exploration of the future of clinical hematology/oncology through precision medicine, drug development, clinical trials, and artificial intelligence.
This series intends to be groundbreaking and will push boundaries. Unique to this series will be a discussion of strategic control points for drug developers, precision medicine companies, AI developers, etc., as they seek to gain market share in the clinical hematology/oncology space. Cross comparisons between companies will be made as the underlying principle of this series will be the optimization of patient care.
This series is intended for essentially everyone, including all healthcare practitioners, anyone affiliated with the pharmaceutical industry, drug developers, data scientists, computational biologists, investors, healthtech employees, etc.
I hope you'll join us for this unprecedented tour of bona fide translational medicine, where the worlds of drug development, precision medicine, data science, and clinical hematology/oncology collide.
Prelude: Conducting a Cellular Symphony with Life Saving Combination Therapies: The global analysis of cells required to optimize combination therapies.
Article 1: Playing Chess Against Cancer: A pharmaceutical, biotechnological, and clinical guide to modern-day oncologic treatment cartography.
Article 2: Quarterbacking a Patient's Fight Against Cancer: If the long-term approach to cancer is a game of chess, the short-term approach is a game of American football.
Article 3: Remember to be Precise: A statistical guide to assessing claims and clinical utility of healthtech AI. We will assess cell-free DNA in population cancer screening, and perform a comparative analysis/strategic control points of associated companies.
Article 4: Comprehensive Precision. We will introduce multiomics inundating the medical forum.
Article 5: An Evolving Battlefield: We will explore the genomics market, and perform a comparative analysis of companies in this space. We will address how genomics companies must avoid becoming Blockbuster in a Netflix world.
Article 6: Too Big to Fail and Too Big to Succeed: We will focus on precision proteomics, including its numerous iterations and associated companies, as we delineate their future clinical relevance.
Article 7: We Hardly Knew You: We will do a deep dive on RNA and explore the world of non-coding RNAs and RNAomics.
Article 8: How to Make a Crystal Ball: We will focus on immunotherapy prognostic assays to better predict immunotherapy response. This includes a comparative analysis of competitors in this space.
Article 9: Avoiding Obsolescence: We will look at the rest of multiomics and discuss their implications in hematology/medical oncology. We will revisit the notion of "comprehensive precision" introduced in article 4.
Article 10: The Cellular Revolution: An exploration of the rapidly evolving cellular therapy world. We will discuss their positioning in hematologic malignancies and solid tumors, and how community physicians will need to adapt or risk being shut out of these malignancies. We will discuss novel chimeric antigen receptor endeavors involving B-cells, neutrophils, macrophages, etc.
Article 11: Predicting the Unpredictable: We will focus on game theory in clinical trial design, positioning of assets, drug development, etc.
Article 12: A Change is Coming: A focus on the present and future clinical "forest" comprised of trees discussed in previous articles.
References
2. https://www.pinterest.ca/pin/434738170264190412/
3. https://howtheyplay.com/team-sports/How-To-Play-American-Football-for-Beginners
4. https://ew.com/movies/2020/01/22/any-given-sunday-oral-history/
7. https://www.si.com/nfl/2017/01/18/david-tyree-giants-where-are-they-now
8. https://www.cnbc.com/2020/09/14/the-worst-job-tom-brady-had-before-he-made-it-to-the-nfl.html
9. https://www.liveabout.com/what-is-an-offensive-lineman-1336341
10. Koba T, Kijima T, Takimoto T, Hirata H, Naito Y, Hamaguchi M, Otsuka T, Kuroyama M, Nagatomo I, Takeda Y, Kida H, Kumanogoh A. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation: Two Case Reports. Medicine (Baltimore). 2017 Feb;96(6):e6087. doi: 10.1097/MD.0000000000006087. PMID: 28178168; PMCID: PMC5313025.
12. Scherer, A. Best Practices for NGS-Based Cancer Diagnostics. Journal of Precision Medicine. 2022.
13. https://leagueside.com/chances-of-going-pro/
14. https://www.businessinsider.com/what-the-inside-of-an-nfl-playbook-looks-like-2011-8
15. Callahan MK, Chapman PB. PD-1 or PD-L1 Blockade Adds Little to Combination of BRAF and MEK Inhibition in the Treatment of BRAF V600-Mutated Melanoma. J Clin Oncol. 2022 May 1;40(13):1393-1395. doi: 10.1200/JCO.21.02801. Epub 2022 Jan 14. PMID: 35030031; PMCID: PMC9061142.
21. https://bleacherreport.com/articles/2039934-nfl-101-introducing-the-basics-of-cover-2
22. https://brobible.com/sports/article/nfl-minimizing-pass-interference-penalty/