Playing Chess Against Cancer: A Pharmaceutical, Biotechnological, and Clinical Guide to Modern Day Oncologic Treatment Cartography
We are witnessing a revolution in cancer therapeutics that is truly bewildering. The number of novel drugs with unique mechanisms of action currently in clinical trial and development is staggering, and will only increase. Much of this has been precipitated by exponential advances in computational biology and precision medicine. To this end, many oncologic breakthroughs in the future will occur at the intersection between these disciplines and the clinic. Thus, understanding the putative clinical relevance and utility of drug development and precision medicine endeavors is essential for related companies, academic researchers, etc. Failure to derive a sophisticated appreciation of how one's work will translate into the clinic can be catastrophic, as you can have "positive related studies" with no clinically applicable "end-game" or return on your investment. Failure of oncologists to develop a strategy for assimilating molecular data and novel agents they will be inundated with in the future will compromise patient care. In this bimonthly editorial series, we strive to ensure readers have clear visibility of the "clinical forest", and not just the associated "trees". We will focus on the present and the future of precision medicine as it relates to the clinic, and provide those in the pharmaceutical industry with an instrumental clinical perspective. We start by introducing the notion of "playing chess against cancer" through treatment cartography, the development of patient specific treatment maps incorporating conventional therapies, clinical trials, molecular studies, etc.
Image credit: Adrienn Harto
In September of 2021, I gave an eight-hour lecture series to the Roswell Park Cancer Institute fellows. It was unconventional as I didn't teach them what I thought they could obtain from journal articles, National Comprehensive Cancer Network (NCCN) guidelines, etc. I focused on conveying a thought process to them. Indeed, as I reflected on my instruction during my training and what I've learned over the years, it occurred to me nobody really teaches you HOW to think while they're teaching you WHAT to think.
The initial concept I emphasized to the Roswell fellows was that they needed to play chess, not checkers, against cancer. They needed to anticipate the best- and worst-case scenarios as they treated their patients and have contingency plans ready at all times. They needed to become experts at "treatment cartography", a term I coined referring to the development of patient specific treatment maps.
I explained to the fellows that when playing chess against cancer it's important to identify the pieces available to you and to think several moves ahead. Accordingly, the very first time I meet a patient who has a theoretically incurable cancer I consider what my entire short- and long-term therapeutic approach will be. In patients with "terminal" cancers we typically use a "first-line" treatment until the cancer progresses, or the patient becomes intolerant to it. Subsequently, we use a "second-line" treatment, and so on and so forth (figure 1). Accordingly, in the very moment I meet a patient with terminal cancer I usually know what my next four-plus treatments will be. I've mapped out as many as 15 therapies for a patient with metastatic hormone-receptor positive, HER-2-negative, breast cancer at their first visit.
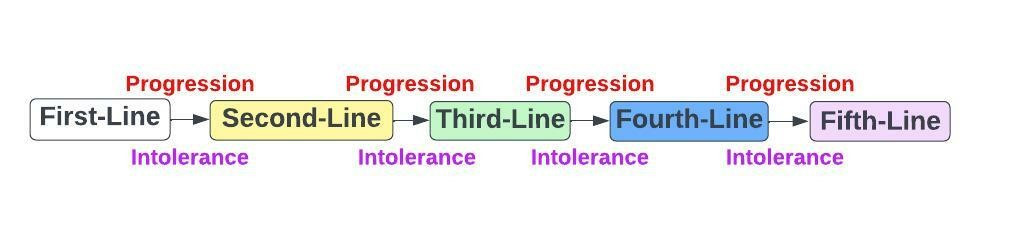
Figure 1: Sequencing Therapies in Terminal Cancers
One of the reasons it's important to define a patient's treatment map at the onset is because failing to do so can render patients ineligible for clinical trials that may benefit them later. Specifically, giving a therapeutic agent to a patient in first-line may exclude them from participating in a trial testing a novel drug in later lines of therapy. For example, consider clinical trial NCT04337970 exploring the efficacy of talazoparib and axitinib in patients with previously treated metastatic clear cell renal cell cancer. One of the trial exclusion criteria is that patients must not have received axitinib previously. To this end, typical first-line treatments used in metastatic clear cell renal cell cancer are [1]:
- Axitinib plus pembrolizumab (low-risk or intermediate/high-risk disease)
- Cabozantinib plus nivolumab (low-risk or intermediate/high-risk disease)
- Lenvatinib plus pembrolizumab (low-risk or intermediate/high-risk disease)
- Ipilimumab plus nivolumab (intermediate/high-risk disease)
One can easily argue that the first three regimens listed are interchangeable as they employ a vascular-endothall growth factor receptor (VEGFR) inhibitor (axitinib, cabozantinib, or lenvatinib) and PD-1 inhibitor (pembrolizumab or nivolumab). However, only the use of axitinib in first-line precludes the patient from enrolling in the talozoparib plus axitinib second-line trial. Thus, it's imperative one consider this immediately upon meeting a patient with newly diagnosed metastatic renal cell cancer if there is any consideration of enrolling the patient in the aforementioned trial later. In this case I would use ipilimumab, a CTLA-4 inhibitor, plus nivolumab, a PD-1 inhibitor, in first-line, assuming the patient had intermediate/high-risk disease, and axitinib, a VEGFR inhibitor, plus talozoparib, a polyADP-ribose (PARP) inhibitor, in second-line via clinical trial. This enables me to "show" the tumor four methods of attack in the first two-lines of therapy, as opposed to any other sequencing of the aforementioned treatments.
Aside from rendering patients ineligible for potential clinical trials, failure to properly sequence a patient's therapeutic options can result in suboptimal long-term outcomes. Using a treatment with minimal relative efficacy in first-line can result in a patient's disease progressing so profoundly they become ineligible for any subsequent therapies. This can be due to cancer related deterioration of a patient's condition, an associated decrease in the patient's kidney, liver, or hematologic function, etc. Accordingly, proper sequencing of therapeutic modalities can constitute the difference between a patient living 3 months or 3 years.
When formulating a patient's treatment map, I typically group available therapeutic options (chess pieces) into one of five broad categories (figure 2) using a COMET acronym I developed, including:
- Conventional systemic therapies that are FDA approved
- Operational (surgical) procedures
- Molecular based options
- Everything else
- Trials testing experimental therapeutics

Figure 2: COMET acronym used in treatment cartography
Potential enrollment in hospice is considered in the context of the COMET rubric.
Conventional systemic therapies refer to all related FDA-approved systemic chemotherapies, biologic therapies, vaccine-based treatments, cellular therapies, radioisotopes, etc.
Operational considerations include resection of metastatic lesions (metastasectomy), liver transplant in metastatic colon cancer patients with liver restricted metastases, tumor debulking procedures, etc.
Molecular based options are delineated by performing next generation sequencing (NGS), PD-L1 immunohistochemistry, and homologous recombination deficiency (HRD), microsatellite instability (MSI), and tumor mutation burden (TMB) testing on the tumor of interest.
NGS, which will be discussed in more detail in a later article, can identify actionable mutations in the tumor that can be targeted with available therapeutic agents, sometimes irrespective of whether or not they're already FDA approved for the tumor of interest.
HRD testing identifies patients who may be candidates for poly(ADP-ribose) polymerase (PARP) inhibitors. PD-L1, MSI, and TMB testing is crucial to identify patients likely to respond to immunotherapy, as tumors with high levels of any of these generally have a higher propensity to do so.
Everything else refers to locoregional therapies including liver-directed treatments (e.g., radioembolization, radiofrequency ablation, microwave ablation, transarterial chemoembolization, etc.), hyperthermic intraperitoneal chemotherapy (HIPEC), radiation (e.g., external beam radiation therapy, stereotactic body radiation therapy), cryoablation, etc.
Trials references consideration of all clinical trials the patient is eligible for, not just those based on their molecular profile. Importantly, a thorough assessment of putative trial efficacy based on the mechanism of action of the novel agent being tested, previous trials conducted in the tumor of interest, standard of care, etc., are crucial to postulating the best clinical trials available to a patient. Unfortunately, there are many trials currently conducted that are overtly nonsensical either in terms of design, drug being tested, etc.
With the COMET rubric in mind consider my 61-year-old female patient who presented with stage 4, left-sided, KRAS, HRAS, NRAS, and BRAF wild-type, sigmoid colon cancer widely metastatic to the peritoneum, liver, and lungs (figure 3).
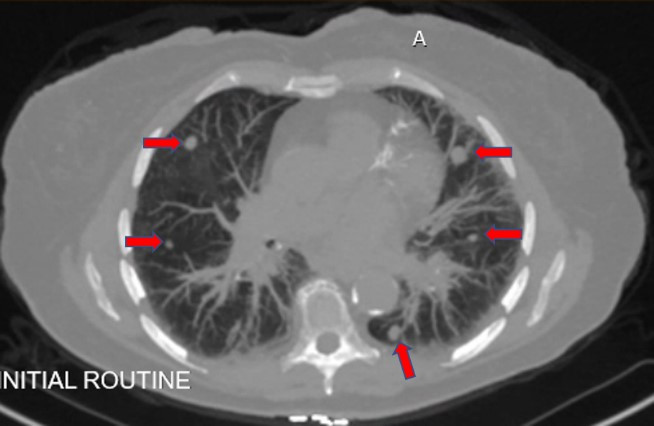
Figure 3: Bilateral lung metastases (red arrows)
The preferred conventional systemic therapies I typically consider in this patient with metastatic colon adenocarcinoma that is left-sided (sigmoid colon), and doesn't harbor a mutation in KRAS, HRAS, NRAS, or BRAF (more on this later) include:
- FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) + panitumumab
- FOLFIRI (5-fluorouracil, leucovorin, irinotecan) + bevacizumab
- Trifluridine/tiperacil (from now on referred to as lonsurf)
- Regorafenib
These treatments are commonly used and supported by high-level efficacy and toxicity related evidence, and NCCN guidelines. However, this isn't dogmatic as there are numerous correct ways to treat metastatic colon cancer. For example, some oncologists use bevacizumab instead of an EGFR inhibitor, such as panitumumab, in conjunction with FOLFOX. Regardless, for simplicity, we will consider the patient's conventional systemic therapies to be aforementioned. Based on this, assuming the patient had no other viable treatments available, I would sequence her treatment as follows (figure 4):
- First-Line: FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) + panitumumab
- Second-Line: FOLFIRI (5-fluorouracil, leucovorin, irinotecan) + bevacizumab
- Third-Line: Lonsurf
- Fourth-Line: Regorafenib
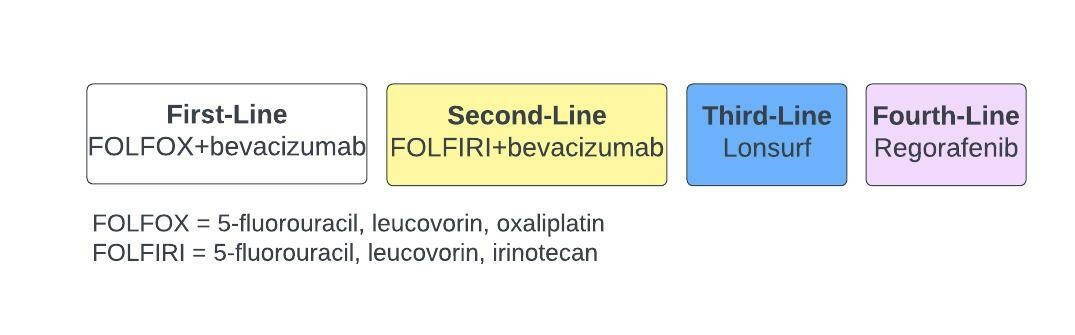
Figure 4: Sequencing of conventional therapies for our patients
The patient is a candidate for an EGFR inhibitor, panitumumab or cetuximab, because she doesn't have a mutation in KRAS, HRAS, NRAS, or BRAF, other than BRAF V600E, and her tumor is left-sided; right-sided colon cancers are considered unresponsive to EGFR inhibitors [2]. Although we will discuss molecular characterization of tumors in more detail later, it's worth mentioning how such tests delineate if metastatic colon cancer patients are candidates for EGFR inhibitors.
EGFR is one of many receptor tyrosine kinases (RTKs). It is situated on the plasma membrane and activated by binding its ligand, EGF (figure 5) [3]. After binding with the ligand EGFR forms homo- and heterodimers with other receptor tyrosine kinases, leading to autophosphorylation of the receptor [4]. This activates the mitogen-activated protein (MAP) kinase and phosphoinositide 3-kinase (PI3-K) pathways, among others (figure 5) [5]. In an incredibly oversimplified MAP kinase pathway iteration, EGFR activation leads to sequential activation of RAS, RAF, MEK, and ERK (figure 5) [5]. Some metastatic colon cancers harbor activating mutations in HRAS, NRAS, KRAS, or BRAF, resulting in constitutive activation of the respective proteins and downstream ERK signaling irrespective of EGFR [6]. Using an EGFR inhibitor that acts upstream of these proteins to inhibit EGFR, such as panitumumab in figure 5, does not account for the constitutive activation of H-, N-, or KRAS, or BRAF, although we do use cetuximab, an EGFR inhibitor, with encorafenib, a BRAF V600E inhibitor, in patients with BRAF V600E mutations [7]. Consequently, colon cancer patients who have mutations in H-, N-, or KRAS, or BRAF, other than V600E, are ineligible for EGFR inhibitors [10]. We test for these mutations in all patients with metastatic colon cancer to see if they can benefit from the FDA approved EGFR inhibitors, cetuximab and panitumumab, in stage 4 colon cancer.
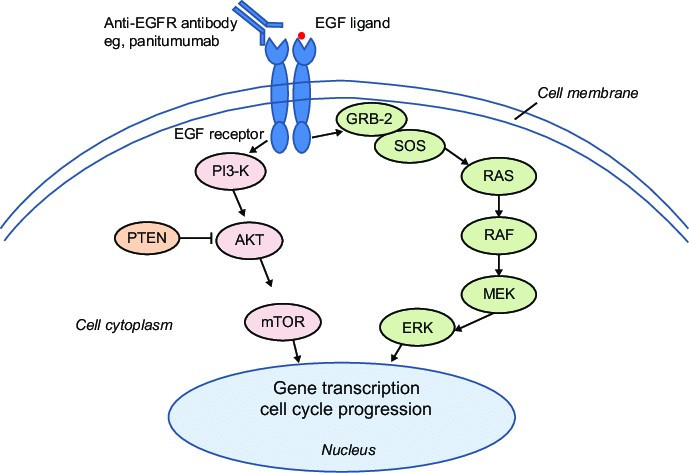
Figure 5: A simple rendition of the EGFR pathway
Now that we've identified the conventional treatment options available to our patient, let's consider the next part of the COMET acronym, surgical (operational) options. Unlike many other stage 4 malignancies (e.g., breast, esophageal, pancreatic) surgery is a mainstay in stage 4 colon cancer. Patients with limited liver [11-13] and/or lung metastases [14-16] may be candidates for liver and/or lung resections with curative intent. In addition, some patients with a sizeable tumor burden that is restricted to the liver undergo curative liver transplantation [17]. Debulking surgeries, in which tumor metastases are resected with palliative intent, are potentially beneficial in metastatic colon cancer [18]. Ultimately, in our patient, due to the diffuse nature of her disease and its involvement of the liver, lungs, and peritoneum, there is no viable surgical option.
The M of COMET refers to molecular based options. Although it didn't use to be, it is very common practice for oncologists to molecularly characterize nearly all stage 4 tumors. This typically involves performing next generation sequencing (NGS), PD-L1 immunohistochemistry, and homologous recombination deficiency (HRD), microsatellite insatiably (MSI), and tumor mutation burden (TMB) testing on the tumor of interest.
NGS allows for the identification of actionable mutations in the cancer, for which targeted therapies are sometimes available. In metastatic non-small cell lung cancer targeted therapies are readily used in cancers with ROS1 translocations, NTRK fusions, RET fusions, ALK translocations, HER2 overexpression, and MET exon 14 skipping, EGFR, KRASG12C and BRAF V600E mutations. In metastatic bladder cancer FGFR2 and FGFR3 fusions or FGFR3 mutations allow for the use of erdafitinib, that inhibits FGFR2 and FGFR3. In metastatic cholangiocarcinoma FGFR2 fusions prompt the use of FGFR2 inhibitors, pemigatinib or infigratiniab, and IDH1 mutations necessitate the use of ivosedinib, an IDH1 inhibitor. In acute myeloid leukemia FLT3 ITD mutations trigger use of FLT3 inhibitors, including midostaurine and gilteritinib, IDH1 mutations prompt the use of ivosedinib, and IDH2 mutations suggest responsiveness to the IDH2 inhibitor, enasidenib. BRCA 1 and 2 mutations, although implicated in homologous recombination deficiency, can be detected qualitatively via next generation sequencing and prompt the use of PARP inhibitors. Indeed, the list of mutations we presently search for across the hematology and medical oncology forums, and their respective targeted therapies, is rapidly expanding and a topic we fully explore in later articles in this series.
The NGS results for our patient are depicted in figure 6.

Figure 6: Molecular Studies performed on our patient's cancer
Importantly, PD-L1 immunohistochemistry, not depicted in figure 6, was negative as none of the patient's cancer cells expressed PD-L1.
The seasoned oncologist will immediately recognize the number of mutations this patient's tumor harbors is striking. Moreover, the composition of those mutations is incredibly rare across all tumors, let alone metastatic colon cancer. Specifically, the patient has actionable mutations in ATM, PTCH1, ARID1A, and RAD50, and an NTRK1 fusion, that are readily targetable with available therapeutics. Specifically, ATM, ARID1A, and RAD50 mutations contribute to homologous recombination deficiency, suggesting that PARP inhibitors, such as olaparib, rucaparib, niraparib, veliparib, and talozoparib, may be useful. There is an activating mutation in PTCH1, an integral component of the Hedgehog pathway, suggesting a Hedgehog inhibitor, such as vismodegib, glasdegib, or sinodegib, may be useful. The NTRK1 fusion represents one of two "holy grails" in this case. Specifically, it is present in 0.2-1% of all metastatic colon cancer patients and allows for the use of NTRK inhibitors [19], such as entrectanib or larotrectinib. These drugs are associated with 75% response rates in tumors harboring NTRK fusions, and have median durations of response in excess of 4 years [20]. The MSH6 mutation is indicative of underlying Lynch syndrome, a hereditary condition that predisposes patients to colorectal cancer, endometrial cancer, bladder cancer, etc. The MYC mutation, although intriguing, isn't presently actionable although there are several companies targeting MYC in various fashions in clinical trial. For example, Vicerx Pharma, Inc. is employing a CDK9 inhibitor to abrogate MYC activation in several ongoing studies. The remaining mutations in our patient's NGS, although interesting, don't presently have FDA approved therapies targeting them. Nonetheless, based on the patient's NGS she is a candidate for:
- PARP inhibitors: olaparib, talozoparib, rucaparib, niraparib, veliparib
- Hedgehog inhibitors: vismodegib, glasdegib, sinodegib
- NTRK inhibitors: larotrectanib, entrectanib
A cornerstone of molecular characterization of tumors entails characterization of the tumor's PD-L1 expression, microsatellite instability (MSI), and tumor mutation burden (TMB) to determine the likelihood of immunotherapy response. PD-L1 is a protein cancer cells express on their cell surface that bind to PD-1 on T-cells, thereby inhibiting them from eliminating the tumor cell (figure 7, left) [21].
Many current immunotherapies abolish the interaction between PD-L1 and PD-1 by binding to either protein, relieving tumor cell inhibition of the T-cell and facilitating immune attack of the cancer cell (figure 7, right). Numerous PD-L1 inhibitors (durvalumab, atezolizumab) and PD-1 inhibitors (pembrolizumab, nivolumab, avelumab, cemiplimab, dostarlimab) are presently FDA approved. In some tumors, such as non-small cell lung, the higher the tumor expression of PD-L1 the more likely a tumor will respond to immunotherapies targeting PD-L1 or PD-1 [22]. In the case of our patient her tumor didn't express PD-L1.
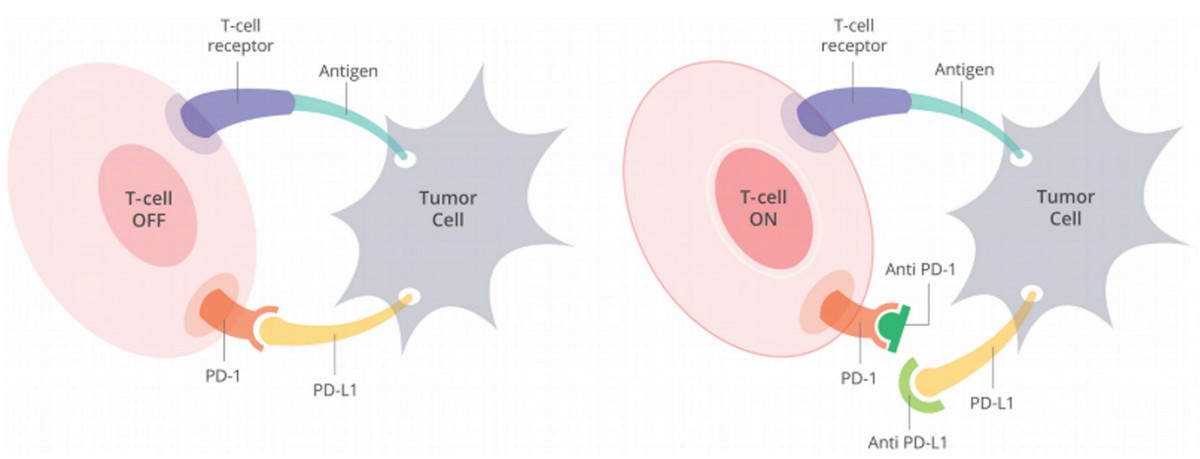
Figure 7: Expression of PD-L1 on tumor cells inhibits T-cells
A thorough description of MSI and TMB will be provided in future articles. Regardless, MSI and TMB are effectively measures of tumor cell DNA instability and mutation burden, respectively. Numerous studies have demonstrated that high microsatellite instability [23] and/or tumor mutation burden [24-27] results in the formation of neoantigens that facilitate immune attack of the tumor cell. As such, tumors that are MSI-high [28] and/or TMB-high [29] have a higher likelihood of being responsive to immunotherapies that activate the immune system, such as PD-1 and PD-L1 inhibitors. Ipilimumab, an immunotherapy targeting CTLA-4, is approved in combination with nivolumab for metastatic colon cancer patients who are MSI-high, as well as numerous other tumors. In what constitutes the second "holy grail" in this case, our patient's tumor was found to have a high TMB (55 mutations/megabase-Figure 6) and was MSI-high (Figure 6), suggesting she will very likely respond to immunotherapy; only about 5% of metastatic colon cancers are MSI-high. As such, FDA approved immunotherapy drugs available to our patient based on her molecular profile are nivolumab alone, dostarlimab alone, pembrolizumab alone, and ipilimumab + nivolumab.
Homologous recombination, a full description of which will be presented in a future article, is one of several mechanisms cells employ to repair DNA damage [30]. It's well established that tumors deficient in homologous recombination are often more susceptible to PARP inhibitors [31], a phenomenon we will explore more in later articles. Clinicians assess for homologous recombination deficiency (HRD) with quantitative homologous recombination assays provided by companies such as Myriad, or qualitatively by searching for mutations in genes involved in homologous recombination, via NGS. As above, this patient's tumor has numerous actionable mutations in genes that contribute to homologous recombination deficiency, including ATM, ARID1A, and RAD50. Thus, she is a candidate for PARP inhibitors, including olaparib, nilipirib, veliparib, talozoparib, and rucaparib.
Combining our molecular based analysis with available conventional treatment options (figure 8), therapies available to our patient include:
- Conventional systemic therapies: FOLFOX+panitumumab, FOLFIRI+bevacizumab, lonsurf, and regorafenib
- Operational: None
- Molecular:
- PARP inhibitors: olaparib, talozoparib, rucaparib, niraparib, veliparib
- Hedgehog inhibitors: vismodegib, glasdegib, sinodesigib
- NTRK inhibitors: larotrectanib, entrectanib
- Immunotherapy: nivolumab, dostarlimab, pembrolizumab. and ipilimumab + nivolumab
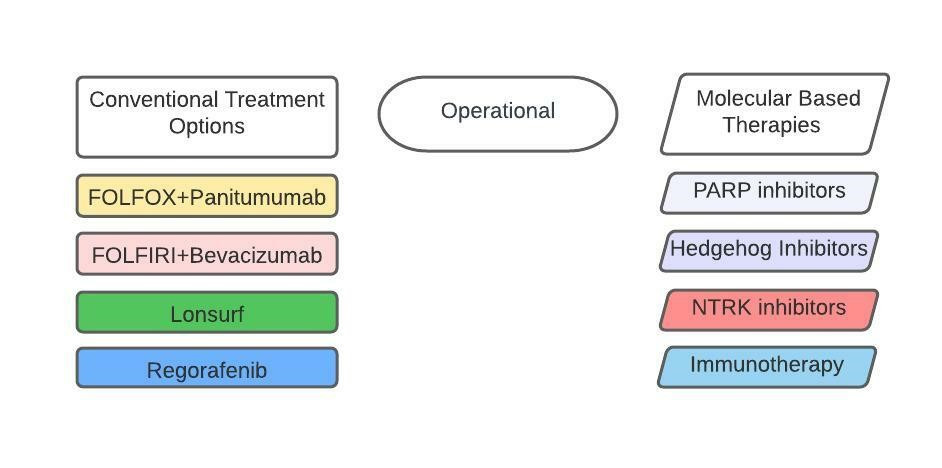
Figure 8: Available treatment options for our patient thus far
We've reached the Everything Else component of the COMET acronym. Here we're referring predominantly to locoregional therapies including, but not limited to, stereotactic body radiation therapy (SBRT), external beam radiation therapy (EBRT), transarterial chemoembolization (TACE), radioembolization (e.g. yttrium 90, SIR-Spheres), HIPEC, cryoablation, microwave ablation, radiofrequency ablation (RFA), etc. (figure 9). Many of these treatment modalities are used in metastatic colon cancer. Specifically, yttrium-90 is commonly administered to patients with liver predominant metastases that aren't surgical candidates [32]. SBRT is used in oligometastatic disease [33]. HIPEC is sometimes administered for peritoneal metastases [34]. In this case, due to the tumor involving the lungs, peritoneum, and liver, none of these treatment modalities are classically indicated at treatment onset for our patient. However, it's conceivable the patient's tumor could become amenable to one of these treatments in the future.

Figure 9: A list of some locoregional techniques used in stage 4 colon cancer
The final component of the COMET algorithm is Trials. Clinical trials are studies often testing experimental therapeutics in patients. They can sometimes constitute the difference between life and death for a patient. However, not all clinical trials are created equal. Some trials are counterintuitive or potentially detrimental to patients. Others intuitively have a minimal chance at success, or obviously will not truly move the needle for a patient. Specifically, drugs employing similar mechanisms of action to drugs that have failed in a given tumor are unlikely to yield a positive result. Moreover, drugs with similar mechanisms of action to already approved therapies for the tumor of interest are unlikely to drastically ameliorate a patient's outcome.
In the first-line chronic lymphocytic leukemia (CLL) GLOW study testing the combination of venetoclax plus ibrutinib versus chlorambucil plus obinutuzumab in elderly patients with CLL, patients on the control arm (chlorambucil plus obinutuzumab) received inferior treatment to standard of care single agent ibrutinib.
Trials employing single agent tivozanib, now FDA approved, that predominantly targets VEGFR, in relapsed/refractory metastatic clear cell renal cell cancer (RCC) were unlikely to markedly improve patient outcomes relative to the other six already approved VEGFR-targeting inhibitors in metastatic RCC. To this end, when considering clinical trials for patients, clinicians must consider numerous factors, many of which are beyond the scope of this article.
Sadly, many clinical trials in metastatic colon cancer have, and still do, leave much to be desired. In fact, since the approval of lonsurf in 2015, no drug has been approved in colon cancer outside of immunotherapy. Presently, intriguing stage 4 colon cancer trials for our patient from my perspective, for various reasons, include:
- NCT04046445: "Phase 1b Study to Evaluate ATP128, VSV-GP128 and BI 754091, in Patients with Stage IV Colorectal Cancer (KISIMA-01)" in second-line.
- NCT05001282: "Phase 1/2 Study to Evaluate ELU001 in Patients with Solid Tumors that Overexpress Folate Receptor Alpha" in all lines, but patients must express the folate receptor alpha.
- NCT05130060: "Phase 1 A Vaccine (PolyPEPI1018 Vaccine) and TAS-102 for the Treatment of Metastatic Colorectal Cancer" in third-line onward.
At this time, we have completed the COMET based approach to our patient. Collectively, the treatment options (figure 10) we elucidated are:
- Conventional: FOLFOX+panitumumab, FOLFIRI+bevacizumab, lonsurf, and regorafenib
- Operational: None
- Molecular:
- PARP inhibitors: olaparib, talozoparib, rucaparib, niraparib, veliparib
- Hedgehog inhibitors: vismodegib, glasdegib, sinodesigib
- NTRK inhibitors: larotrectanib, entrectanib
- Immunotherapy: nivolumab, dostarlimab, pembrolizumab. and ipilimumab + nivolumab
- Everything Else: None
- Trials
- Trial 1) NCT04046445: "Phase 1b Study to Evaluate ATP128, VSV-GP128 and BI 754091, in Patients With StageIV Colorectal Cancer (KISIMA-01)" in second-line onward.
- Trial 2) NCT05001282: "Phase 1/2 Study to Evaluate ELU001 in Patients with Solid Tumors that Overexpress Folate Receptor Alpha" in all lines, but patients must express the folate receptor alpha.
- Trial 3) NCT05130060: "Phase 1 A Vaccine (PolyPEPI1018 Vaccine) and TAS-102 for the Treatment of Metastatic Colorectal Cancer" in third-line onward
*** We are eliminating trial 2 due to the required expression of folic receptor alpha for the purpose of this discussion.
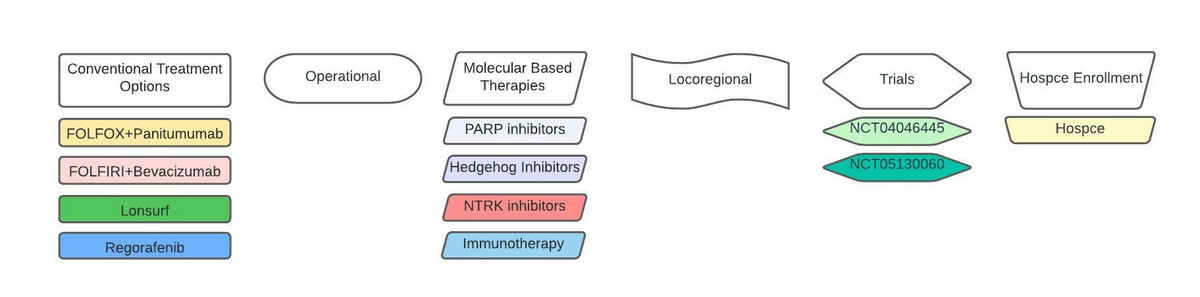
Figure 10: Treatment options available to our patient after completing our COMET approach.
At this juncture, it's time to perform treatment cartography and generate our patient's treatment map. To do this we consider the risk/benefit ratio of each treatment by referencing studies and current treatment guidelines involving each of them in metastatic colon cancer. In instances where there are no studies available pertaining to our molecular based treatments, we use intuition based on mechanism of action, efficacy in similar tumor types, experience, etc., to impute their risk/benefit ratio. Similarly, for our trial options, we attempt to deduce putative efficacy based on numerous criteria, including mechanism of action.
Conventional systemic therapies available to the patient, including FOLFOX+panitumumab and FOLFIRI+bevacizumab, are attractive as they have response rates of about 35-57% with median progression free survival (PFS) of approximately 10 months [35-36]. In contrast, lonsurf alone yields only a 0.3 month median PFS, and 1.8 month overall survival (OS) benefit, respectively [37]. Regorafenib alone is associated with a 1.4 month median OS benefit and only a 0.2 month extension of median PFS [38].
It's well established that colon cancers with high microsatellite instability (MSI-high) are often susceptible to immunotherapy [39-41]. Ipilimumumab plus nivolumab demonstrated an overall response rate of 55%, 1-year PFS of 71%, and 1-year OS of 85% in patients who had MSI-high stage 4 colon cancer [42-44]. Approximately 70-80% of patients with NTRK fusions across all tumor types respond to NTRK inhibitors with a median duration of response of approximately 4 years [20]. However, the data is less compelling in the subset of patients with metastatic colon cancer where overall response rate was about 25-50% [45-47]. We deduce that PARP inhibitors and hedgehog inhibitors, although options for our patient, are unlikely to provide a profound progression free survival or overall survival benefit for numerous reasons we will not expound on here.
When evaluating the two trial options available to our patient we note they employ novel mechanisms of attack the patient's tumor is unexposed to classically. The lonsurf plus PolyPEPI1018 Vaccine trial is appealing as a substitute for lonsurf conventional therapy, as it allows us to target the patient's tumor with an additional mechanism of attack without compromising standard of care. In addition, the trial evaluating ATP128, VSV-GP128 and BI 754091, in second-line onward, is intriguing based on its unique mechanism of action. Of course, every clinical trial evaluation, such as the one performed here, is incredibly subjective. Indeed, one must be very cautious imputing putative clinical trial efficacy as many trial results belie intuition. Regardless, going through the aforementioned clinical trial analysis is a foundational component of treatment cartography.
Synthesizing all of our options, including hospice enrollment, results in creation of the following treatment map (figure 11):
First-line: Immunotherapy in the form of Ipilimumab+nivolumab. Response rates with this regimen are approximately 55% with many individuals having a duration of response longer than two years. Moreover, there is a remote possibility this regimen can effectively "cure" patients by resulting in long-term remissions even after treatment is discontinued. Therefore, we are choosing ipilimumab+nivolumab over all other available options, including pembrolizumab and dostarlimab. However, despite selecting ipilimumab+nivolumab due to the incremental benefit provided by addition of ipilimumab, we would fault nobody for choosing pembrolizumab, nivolumab alone, or dostarlimab.
Second-line: NTRK fusion inhibitor, entrectanib or larotrectanib. One could certainly have chosen conventional therapy in the form of FOLFOX+bevacizumab here. We elected to use an NTRK fusion inhibitor due to it resulting in an overall response rate of 75% and 4.5 year median duration of response across all tumor types. However, we do note in the eight stage 4 colon cancer patients treated with NTRK inhibitors the overall response rate was 50% with far less appealing depth of response [45-47]. Nonetheless, we feel the risk/benefit ratio of NTRK inhibitors, that are generally better tolerated than chemotherapy, warrants use of them in second-line.
Third-line: FOLFOX+panitumumab, After using our two best molecular based options we now turn to conventional therapy. As above, FOLFOX+panitumumab is our conventional therapy of choice and it fits nicely in third-line here.
Fourth-line: FOLFIRI+bevacizumab, We stay with conventional therapy and use our next planned systemic therapy based on known efficacy, particularly compared to our other options.
Fifth-line: Trial 3 sees the light of day as we prefer to use lonsurf+PolyPEPI1018 Vaccine over conventional lonsurf alone, as our patient's tumor will see a novel mechanism of attack while also receiving standard of care.
Sixth-line: Trial 1 is the choice over regorafenib due to the abysmal median overall survival benefit of regorafenib and its marked toxicity. It's also subjectively more appealing than our other molecular based options, PARP- and Hedgehog inhibitors.
Seventh-line: Off label PARP inhibitor. Again, we are very reticent to use regorafenib and would prefer an "outside the box" approach based on the patient's molecular profile. Admittedly, it may be difficult to get insurance to pay for a PARP inhibitor in this setting, and the patient may need to receive it through a precision medicine-based trial.
Eighth-line: Off label Hedgehog inhibitor. We remain reticent to use regorafenib due to its risk/benefit ratio, but aren't particularly enthused about the Hedgehog inhibitor here for similar reasons. As above, it's unlikely insurance would authorize a Hedgehog inhibitor in this setting.
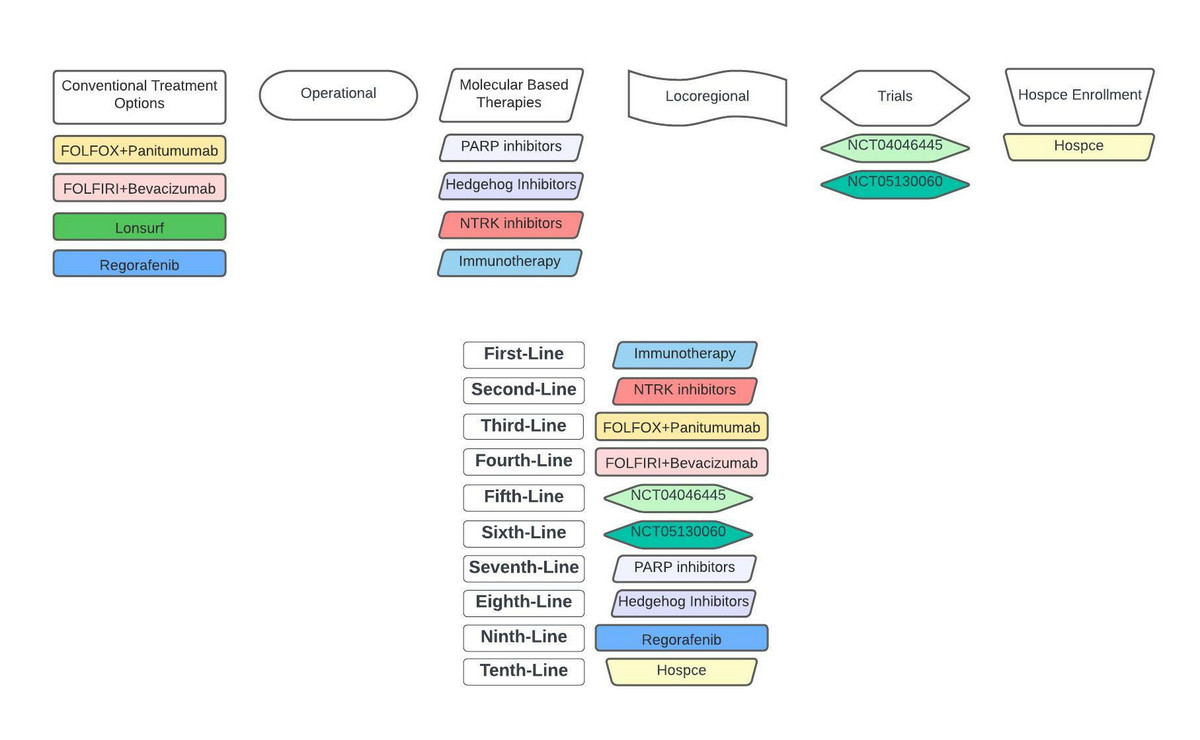
Figure 11: Personalized treatment map for our patient after completing COMET-based treatment cartography.
Ninth-line: Regorefenib. We have run out of therapeutic options in the event the patient can still tolerate therapy and wants to continue treatment.
Tenth-line: Hospice. We simply have no more to offer the patient, and it makes sense to enroll in hospice.
It's important to realize that essentially no stage 4 colon cancer patient is going to receive ten lines of therapy, such as what's listed here. Although it's not uncommon for stage 4 colon cancer patients to receive 3-4 lines of therapy, the majority of patients don't receive 6 plus lines of treatment. Consequently, we desperately need better conventional options in metastatic colon cancer, as our third-line treatments onward are suboptimal. Recognizing this is essential if you're a pharmaceutical company considering how to position a new asset in stage 4 colorectal cancer, a drug developer determining what proteins and tumors to target, a clinician deciding on what clinical trials to pursue, etc.
Many will argue there is no need to have such an intricate approach when initially meeting a patient as the disease isn't static and the treatment approach may change dramatically in the future. Certainly, nothing oncologists do happens in a vacuum. Cancer cells are very good chess players and are almost always several moves ahead of us metaphorically speaking. It's the reason we simply can't cure stage 4 colon cancer that is widely metastatic, despite the fact we can frequently eradicate large swaths of a patient's tumor.
Cancer cells employ numerous treatment resistant mechanisms we just don't understand fully at this time (see the article, "Conducting a Cellular Symphony with Life Saving Combination Therapies" I wrote for biopharmatrend.com). The oncologist needs to be extremely malleable and adaptive, as marked changes in the initial treatment approach may be warranted throughout a patient's treatment. Specifically, new clinical trials may become available that one may use in place of a given therapy. The patient's organ function may change in a manner that precludes use of a given treatment; for example, one cannot give FOLFIRI in a patient with severe liver dysfunction. The patient simply may not tolerate a particular treatment, including 5-fluorouracil, oxaliplatin, etc. New colon cancer molecular targets and associated therapies may be discovered, and need to be incorporated in the treatment paradigm. Nonetheless, engaging in treatment cartography facilitates optimal sequencing of therapies, proper identification of opportunities and unmet needs for pharmaceutical companies, ideal clinical trial design, future directions for precision medicine companies, etc. It's readily obvious looking at our patient's treatment map that an ideal trial for a pharmaceutical company with new drug A would be: FOLFOX+panitumumab +/- drug A to position the asset at the first use of conventional treatment. In fact, it would be better to do FOLFOX+bevacizumab +/- drug A for numerous reasons, but the principle of trying to position an asset where it is most likely to be extensively used is the same.
At Cancer Clarity, a company we recently started, we often assist our pharmaceutical partners with positioning of assets based on the rubric outlined here. Indeed, if the drug being developed has no prospect of being incorporated in patients' treatment maps it is futile to pursue it. To this end, we advise our clients a significant amount of game theory is intrinsic to drug development and clinical trial design, and predicated on treatment cartography. Pharmaceutical companies, drug developers, etc., must have a firm grasp of their "end-game" in the clinical forum prior to embarking on drug development endeavors. We encourage pharmaceutical companies to engage with clinicians, along with key opinion leaders, who have a firm grasp of treatment cartography across all tumor types at the beginning of drug development and clinical trial design. This facilitates optimal development and positioning of assets through global consideration of oncologic unmet needs, treatment paradigms, etc.
Our patient discussed here has a tremendous prognosis due to her tumor being MSI-high, TMB-high, and having an NTRK1 fusion. Whereas median overall survival for all mestatic colon cancer patients is approximately 2.5 years, our patient could very well be expected to live far longer, largely because of her tumor's molecular profile. She was sent for genetic testing to assess for Lynch syndrome due to her MSI-high tumor with a MSH6 mutation, and because all colon cancer patients under the age 70 warrant such testing.
I have played chess my entire life. The best chess player I've ever played to date was cancer. It is almost always several moves ahead, and it often feels like the tumor is cheating; as though it has pieces and moves, I've never seen or heard of. However, I'm confident that with the explosion in hematologic and oncologic drug development, continued evolution of multiomics providing additional molecular based targets, etc., that my win percentage against cancer will dramatically improve in the future. I look forward to watching my colleagues, ranging from clinical oncologists and computational biologists, employ logic similar to what is outlined here to address our most profound hematology/oncology unmet needs.
References
1. National Comprehensive Cancer Network (NCCN) Kidney Cancer Guidelines Version 4.2022.
2. Bahl A, Talwar V, Sirohi B, Mehta P, Arya D, Shrivastava G, Dahiya A, Pavithran K. Primary Tumor Location as a Prognostic and Predictive Marker in Metastatic Colorectal Cancer (mCRC). Front Oncol. 2020 Jun 16;10:964.
3. Yarden Y. The EGFR family and its ligands in human cancer, signaling mechanisms and therapeutic opportunities. Eur J Cancer. 2001 Sep;37 Suppl 4:S3-8.
4. Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem. 2020;20(10):815-834.
5. Tay R, Wong R, Hawkes E. Treatment of metastatic colorectal cancer: Focus on panitumumab. Cancer Management and Research. 2015;7:189-198.
6. Sakata S, Larson DW. Targeted Therapy for Colorectal Cancer. Surg Oncol Clin N Am. 2022 Apr;31(2):255-264.
7. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab a meta-analysis. Eur J Cancer. 2015;51:587-594.
8. Lievre A. Bachatte J-B, Blige V, et. al. KRAS mutation as an independent prognostic factor in patients with advanced colorectal cancer treated with Cetuximab. J Clin Oncol. 2008;26:374-379.
9. Armado IG, Wolf M, Peters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634.
10. Douillard JY, Oliner KS, Siena S, et al. Panitumumab--FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034.
11. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical Management and Outcomes of Colorectal Cancer Liver Metastases. Br J Surg. 2010;97(7):1110–8.
12. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of Long-Term Survival After Hepatic Resection for Metastatic Colorectal Cancer: A Multifactorial Model of 929 Patients. Ann Surg. 2008;247(1):125–35
13. Cummings LC, Payes JD, Cooper GS. Survival After Hepatic Resection in Metastatic Colorectal Cancer: A Population-Based Study. Cancer. 2007;109(4):718–26.
14. Booth CM, Nanji S, Wei X, Mackillop WJ. Outcomes of Resected Colorectal Cancer Lung Metastases in Routine Clinical Practice: A Population-Based Study. Ann Surg Oncol. 2016;23(4):1057–63.
15. Embun R, Rivas de Andrés JJ, Call S, de Olaiz Navarro B, Freixinet JL, Bolufer S, et al. Causal Model of Survival After Pulmonary Metastasectomy of Colorectal Cancer: A Nationwide Prospective Registry. Ann Thorac Surg. 2016;101(5):1883–90.
16. Patel D, Townsend AR, Karapetis C, Beeke C, Padbury R, Roy A, et al. Is Survival for Patients With Resectable Lung Metastatic Colorectal Cancer Comparable to Those With Resectable Liver Disease? Results From the South Australian Metastatic Colorectal Registry. Ann Surg Oncol. 2016;23(11):3616–22.
17. Guidolin K, Choi WJ, Servidio-Italiano F, Quereshy F, Sapisochin G. Attitudes of Canadian Colorectal Cancer Care Providers towards Liver Transplantation for Colorectal Liver Metastases: A National Survey. Curr Oncol. 2022 Jan 28;29(2):602-612.
18. Mishina T, Uehara K, Ogura A, Murata Y, Aiba T, Mizuno T, Yokoyama Y, Ebata T. Role of resection for extrahepatopulmonary metastases of colon cancer. Jpn J Clin Oncol. 2022 Apr 8:hyac045.
19. Ratti M, Grizzi G, Passalacqua R, Lampis A, Cereatti F, Grassia R, Hahne JC. NTRK fusions in colorectal cancer: clinical meaning and future perspective. Expert Opin Ther Targets. 2021 Aug;25(8):677-683.
20. David S. Hong, Lin Shen, Cornelis Martinus van Tilburg, Daniel Shao-Weng Tan, et al. Long-term efficacy and safety of larotrectinib in an integrated dataset of patients with TRK fusion cancer. Journal of Clinical Oncology. 2021 39:15_suppl, 3108-3108.
21. http://blogs.shu.edu/cancer/2016/11/09/how-pd-1-abrogates-the-anti-tumor-immune-response/
22. Davis, A.A., Patel, V.G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. immunotherapy cancer. 2019; 7:278.
23. Xiao, Y., & Freeman, G. J. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer discovery. 2015;5(1):16–18.
24. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348 (6230):124-128.
25. Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):2189-2199.
26. Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207-211.
27. Garofalo A, Sholl L, Reardon B, et al. The impact of tumor profiling approaches and genomic data strategies for cancer precision medicine. Genome Med. 2016;8(1):79.
28. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020 Dec 3;383(23):2207-2218.
29. Cao D, Xu H, Xu X, Guo T, Ge W. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. Oncoimmunology. 2019 Jun 16;8(9):e1629258.
30. O'Sullivan Coyne G, Karlovich C, Wilsker D, et al. PARP Inhibitor Applicability: Detailed Assays for Homologous Recombination Repair Pathway Components. Onco Targets Ther. 2022;15:165-180.
31. Giudice E, Gentile M, Salutari V, et al. PARP Inhibitors Resistance: Mechanisms and Perspectives. Cancers (Basel). 2022;14(6):1420.
32.Entezari P, Gabr A, Salem R, Lewandowski RJ. Yttrium-90 for colorectal liver metastasis - the promising role of radiation segmentectomy as an alternative local cure. Int J Hyperthermia. 2022;39(1):620-626.
33. Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol. 2014;20(15):4220-4229.
34. Yurttas C, Hoffmann G, Tolios A, et al. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J Clin Med. 2018;7(12):567.
35. Botrel, T.E.A., Clark, L.G.d.O., Paladini, L. et al. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677
36. Battaglin F, Puccini A, Ahcene Djaballah S, Lenz HJ. The impact of panitumumab treatment on survival and quality of life in patients with RAS wild-type metastatic colorectal cancer. Cancer Manag Res. 2019;11:5911-5924.
37. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015; 372 1909-1919.
38. Grothey A, Van Custem E, Sobreor A, et al. Regorafenib monotherapy for previously treated metastatic colorecvtal cancer (CORRECT) an international, multicentre, randomized, placebo-controlled phase 3 trial. Lancet. 2013;381:303-312.
39. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
40. Le DT, Kim TW, Van Cutsem E, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer Keynote 164. J Clin Oncol. 2020;38:11-19.
41. U.S. Food & Drug Administration. Package Insert. OPDIVO (nivolumab) injection, for intravenous use. 2020.
42. Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779.
43. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18:1182-1191.
44. Morse MA, Overman MJ, Hartman L, et al. Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist. 2019;24:1453-1461.
45. U.S. Food & Drug Administration. Package Insert. VITRAKVI (larotrectanib) capsules, for oral use. 2018.
46. Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusion. Mod Pathol. 2019;32:147-153.
47. Okamura R, Boichard A, Kato S, et al. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis Oncol. 2018; 2018.