How Pharma Reacts To Wuhan Coronavirus Outbreak
UPDATE April 04. 2020.
The number of confirmed cases of COVID19 approaches 1M globally. Since too many drug repurposing programs and vaccine development projects have been urgently initiated, we decided to summarize them in a separate post:
A Running List Of COVID-19 Treatments And Vaccines In Development
With this, we stop further updates of this news roll, all new COVID research will be reported the Running List -- stay safe and healthy!
UPDATE March 18, 2020:
BREAKING: China says a flu drug favipiravir approved in Japan was effective in a study of 340 coronavirus patients. According to official sources, the drug is effective in mild or moderate cases. Patients treated with favipiravir turned Covid-19-negative after a median of 4 days since the initial positive test -- which is a substantial improvement compared with a median of 11 days for the untreated patients. X-rays examinations confirmed improvements in lung condition in about 91% of the patients treated with favipiravir vs 62% or those without the treatment.
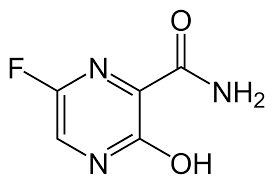
Favipiravir formula
UPDATE March 17, 2020: The first clinical trial for the Wuhan coronavirus (COVID-19) vaccine has started in the US from today on -- for mRNA based vaccine developed through a partnership between the US National Institute of Allergy and Infectious Diseases (NIAID) and Moderna Therapeutics. Notwithstanding the record-breaking speed for this Phase 1 clinical trial to have begun, the vaccine will be available to the general public not earlier than a year from now, in the best-case scenario.
UPDATE March 14, 2020: FDA Approves Roche’s Coronavirus Test, which will speed up testing by an order of magnitude. This is the first commercially-available test which takes around 3.5 hours.
A detailed live statistics for Wuhan coronavirus shows the current number of 155,209 cases, 5,811 deaths, 74,262 recovered. A full list of biotech companies rallying in this space -- at the end of this post.
UPDATE Feb 5, 2020:
- Utah medtech Co-Diagnostics Inc (NASDAQ: CODX), a developer of a test for the Wuhan virus strain
- Integrated DNA Technologies (IDT) has already shipped synthetic genes for use in the pursuit of coronavirus vaccines, and customized oligonucleotide probes and primers to help accurately detect the Wuhan virus.
- Cerus Corporation (NASDAQ: CERS) uses proprietary technology to treat the SARS strain, currently looking to adapt its approach to the novel virus
- Distributed Bio is developing Centivax, a new kind of universal vaccine, but it is unclear how and when it can be applied to the current strain
- GenScript is freely offering to researchers a qRT-PCR detection assay based test for coronavirus, which might be helpful to detect infection early
- Mammoth Biosciences develops a toolbox for the next generation of CRISPR-based diagnostics, partners with SF based researchers developing a precise diagnostics test against coronavirus.
- Sherlock Biosciences is a biotech startup developing CRISPR-based test for coronaviruses -- it requires a blood, urine, or saliva sample for the analytics.
- AbCellera is trying to identify antibodies that can neutralize the virus and block transmission.
UPDATE Feb 04, 2020: A number of biotech companies also join the race to develop treatments or vaccines against Wuhan coronavirus, including US biotech companies such as Regeneron Pharmaceuticals and Vir Biotechnology. Regeneron studies a combination of neutralizing monoclonal antibodies REGN3048 and REGN3051, while Vir is "working to rapidly determine whether its previously identified anti-coronavirus monoclonal antibodies (mAbs) bind and neutralize 2019-nCoV, also referred to as 'Wuhan coronavirus.'" Shares of Vir Biotechnology surged as much as 38% upon announcement to join the race.
Following the coronavirus outbreak, a total death toll raised up to 427, with more than 20.500 infected people confirmed globally.
UPDATE Jan 31, 2020: WHO declared Wuhan coronavirus a global health emergency, hours after the first registered case of human-to-human transmission in the US. A total death toll raised 213, with almost 10,000 cases confirmed globally.
A Wuhan coronavirus outbreak was first reported in early January 2020 and since that time more than 4,500 people have been confirmed to be infected, and 106 dead (actual as of Jan 28th) -- primarily in an 11 million city of Wuhan, the capital of the Chinese province Hubei. More than 70 cases were reported in 17 other places outside China, including at least 5 cases in the US.
What is Wuhan coronavirus?
Wuhan virus (WHO 2019nCoV) is a positive sense, single stranded RNA beta coronavirus, a member of Beta-CoV lineage B (subgenus Sarbecovirus), supposedly able for human-to-human transmission -- according to Zhong Nanshan, head of the health commission team which investigates the outbreak. The RNA sequence is around 30 kb in length.
There is a detailed technical report by Innophore, an Austrian AI-driven biotech company focused on enzyme discovery, providing information about the virus, its structure, active sites, sequence data, Innophore’s early research and modelling experiments, and strategies to develop an efficient antiviral, possibly via repurposing the existing protease inhibitors. The data and ideas are available for public review by researchers across the globe.
While showing similarities to beta coronaviruses found in bats, the new virus is genetically different from other coronaviruses such as the Middle East respiratory syndrome-related coronavirus (MERS), and Severe acute respiratory syndrome-related coronavirus (SARS). There are 18 genomes of the novel coronavirus, that are already isolated and available from the China CDC, Institute of Pathogen Biology, and Wuhan Jinyintan Hospital.
Possible treatments
Currently, there is no specific treatment and vaccine against Wuhan coronavirus. However, if the novel virus was similar to SARS, there would be a potential way to treat patients with ribavirin, protease inhibitor, and interferon.
The Beijing branch of China’s National Health Commission said that a combination of lopinavir and ritonavir is part of its latest treatment plan for those infected by 2019nCoV and suggested taking two lopinavir/ritonavir pills and inhaling a dose of nebulized alpha-interferon twice a day.
According to Michael Mina, an epidemiologist at the Harvard School of Public Health, he has heard about the cases where patients in China are being treated with some level of success with protease inhibitors, originally supposed to treat HIV. Protease inhibitors and some other drugs were also reported by the University of Hong Kong School of Medicine to be successful against SARS, MERS, and other coronaviruses, suggesting repurposing opportunities.
What pharma companies do in response to Wuhan virus outbreak?
While it is still hard to assess a potentially global epidemic risk -- the World Health Organizations has not yet declared a “global health emergency” -- the discovery of efficient treatments, and most importantly, an efficient vaccine against 2019nCoV might take, in theory, from several months to more than half a year. Practically, however, this period will be substantially longer, considering purely manufacturing, logistical and organizational delays of getting sufficient amounts of medications, and vaccines to the remote locations. So the proactive action is essential.
Several pharma companies already raced to explore ways to defend against the novel virus, in collaborations with their Chinese R&D partners. For instance, GeoVax Labs, an Atlanta-based biotechnology company active in immunotherapies and vaccines discovery just signed a letter of intent with Wuhan-based vaccine developer BravoVax to develop a vax against the novel virus. GeoVax will use its MVA-VLP vaccine discovery platform to design and construct the vaccine candidates with the help of genetic sequencing data obtained from the Chinese epicenter of the outbreak. BravoVax will provide development and manufacturing support, as well as lead interactions with local health and regulatory authorities in China.
Another company, Inovio Pharmaceuticals, has been awarded a grant of up to $9 million by the Coalition for Epidemic Preparedness Innovations (CEPI) -- to develop a vaccine. Part of the grant will cover Inovio's preclinical and clinical development through Phase 1 human testing of INO-4800, its new coronavirus vaccine matched to the outbreak strain.
A few days ago, Moderna Therapeutics announced that it had entered into a partnership with CEPI for the same purpose – to come up with an mRNA vaccine to curb the coronavirus.
Maryland-based small biotech Novavax Inc, also announced its efforts towards an anti-2019nCoV vaccine, however, there is an analytics report questioning the company’s ability to quickly bring this yet-to-be-developed product to market.
No matter what the situation is with the actual capabilities and timelines with the vaccine development, stocks for the latter three companies — Inovio Pharmaceuticals, Moderna Inc., and Novavax Inc. — all soared recently driven by the coronavirus-related reports.
Big pharma also jumps in on the Wuhan coronavirus outbreak
For example, AbbVie’s anti-retroviral pill Aluvia (lopinavir/ritonavir), also known as Kaletra, demonstrated repurposing potential to treat the novel virus. Kaletra works by blocking a protease used by the coronavirus to reproduce in the human body. Although the drug was initially developed to block another protease, it may be comparable to gain some positive effect to delay disease progression. AbbVie donated over $1M worth of medication to help treat the Chinese epidemic crisis.
In coordination with researchers and clinicians in the U.S. and China, Gilead Sciences is studying its experimental Ebola treatment remdesivir as a possible repurposed drug candidate against Wuhan coronavirus.
Finally, there is Johnson & Johnson who also jumped in on coronavirus vax discovery work.
Concluding this post, there is certainly a growing hype and panic in the media, but it is still too early to form an opinion about the real risk of the global epidemy. However, this situational trend will certainly bring opportunities to a number of niche biotech players, as well as big pharma companies focused on antivirals. The important thing, there are repurposing options among drugs, originally emerged during the earlier coronavirus outbreaks in 2003 and later. Some lessons have already been learned at that time so now there might be shortcuts to develop medications and vaccines against 2019nCoV faster.
