Sponsored by Lantern Pharma
AI Breaches the Barrier Towards Better CNS Drug Discovery
The sphere of oncology continues to grapple with the complex nature of brain and central nervous system (CNS) tumors. Data predicts that in 2023, the United States will witness approximately 24,810 adults and 5,230 children diagnosed with primary brain or spinal cord tumors. When viewed on a global scale, the figures from 2020 reveal that over 308,000 individuals confronted the reality of such primary tumors. Notwithstanding the advancements in therapeutic strategies, the fatality rate associated with brain and other nervous system cancers persistently occupy the tenth position in the list of global causes of death. The leading impediment to developing effective treatments can be traced back to the brain's intricate layout and the unique properties of blood-brain barrier.
What is the blood-brain barrier and why is it a challenge?
The blood-brain barrier (BBB) is a specialized system of filtering and regulation within the brain's blood vessels. It functions as a selective gatekeeper, letting beneficial substances in while keeping harmful ones out. The barrier is composed of tightly packed endothelial cells, which create an almost impenetrable shield, making it very difficult for substances to pass without assistance. Some molecules can permeate this barrier if they're small or lipid-soluble, as the cell membranes of the BBB are lipid-based. However, larger or water-soluble molecules, including vital nutrients, typically require a form of transportation to cross the BBB. This sophisticated structure of the BBB is vital for maintaining brain health and function.
The blood-brain barrier poses a formidable obstacle in the pursuit of effective medications for brain and CNS cancers. Functioning as a rigid, discerning filter, the BBB denies entry to approximately 98% of small molecule drugs and all macromolecular therapeutics, restricting the reach of potential treatments for these complex conditions.
A recent examination of Phase III clinical trials has found an alarming trend: out of 446 such studies focusing on advanced solid tumors, as many as 169 (or 36.4%) completely turned away patients suffering from brain metastases. Additionally, another 140 trials (or 30.2%) applied conditional exclusions, demonstrating an entrenched hesitance towards involving these patients, primarily due to worries about potentially low efficacy of systemic agents due to low permeability of the blood-brain barrier,
In addition, current methodologies for evaluating BBB permeability impose added complexities and costs. Traditional practices typically revolve around wet-lab experiments that are not only extremely labor-intensive and lengthy but also carry substantial financial burden. The BBB's unique and stringent selectivity often necessitates the implementation of specialized Phase 0 trials in CNS oncology programs. Because of BBB, these trials require higher systemic drug concentrations to achieve detectable levels within the CNS, as compared to other therapeutic areas, increasing the complexity and risk of the process. Moreover, the absence of reliable biomarkers for assessing drug activity in the brain further compounds these issues.
Thus, the twin challenges of the blood-brain barrier’s inhibitive selectivity and the cost and complexity of permeability testing form a significant bottleneck in the field of oncology drug discovery. Those obstacles restrict the advancement of potential therapeutic candidates, underscoring the urgent need for an innovative, accurate, and cost-effective solution to tackle the BBB conundrum and push forward the frontier of treatments for brain and CNS cancers.
New Hope for CNS Cancer Treatment
Recently, Dallas-based artificial intelligence (AI)-driven drug discovery company Lantern Pharma (NASDAQ: LTRN) announced a significant breakthrough in addressing the challenge of the blood-brain barrier in CNS cancer research. Using their novel highly accurate AI algorithms, they have managed to predict BBB permeability with an impressive 89-92% accuracy, offering a rapid, cost-effective way to screen drugs or compounds to determine their potential to cross the BBB. This advancement is pivotal in accelerating the development of drug candidates for brain and CNS cancer patients.
In a testament to the exceptional capabilities of Lantern Pharma’s AI-driven RADR® platform, the Therapeutic Data Commons (TDC), a leading evaluator of therapeutic-focused AI algorithms, ranked all four of Lantern's BBB permeability prediction algorithms as the best in the field.
The newly-developed BBB permeability prediction AI algorithms are fully integrated into Lantern’s AI platform RADR® and are being made available to Lantern’s recently founded wholly-owned subsidiary, Starlight Therapeutics, to further advance its brain and CNS cancer drug programs.
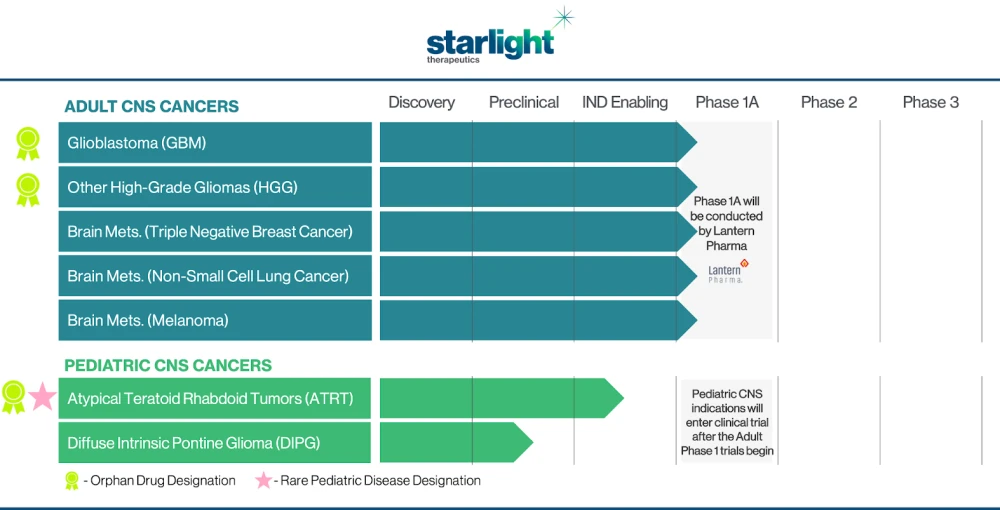
Starlight Therapeutics Pipeline of Adult and Pediatric CNS Cancer Indications for their Drug Candidate STAR-001
Starlight Therapeutics is solely dedicated to the clinical development of treatments for CNS and brain cancers, particularly those where effective therapeutic options are currently limited or nonexistent. Their prime AI-developed drug candidate, STAR-001, stands out as it has demonstrated permeability through the blood-brain barrier, favorable bioavailability in brain tumors, and exhibited nanomolar potency across a wide array of in-vitro and in-vivo CNS and brain cancer models. The company is looking forward to initiating clinical trials for STAR-001 in adult and pediatric CNS cancer indications by late 2023 or early 2024.
Advancing precision oncology with AI
The newly developed blood-brain barrier permeability predictive AI algorithm is only one facet of the RADR® platform, which also offers a comprehensive suite of solutions for oncology drug discovery. It harnesses over 25 billion clinical data points, 154+ drug-tumor interactions, and 130,000+ patient records from diverse resources, including public datasets, commercial clinical studies, and Lantern's 3D tumor models. This comprehensive tool uses this multi-omics data to predict drug responses, generating candidate biomarkers, and aiding in patient stratification for clinical trials.
RELATED: Unveiling Lantern Pharma's Success Story in AI-powered Precision Oncology
More than just refining patient selection, RADR® bolsters risk mitigation and success optimization in drug development by enhancing patient enrichment, potentially reducing research costs, hastening therapy delivery, and personalizing treatments. The platform’s operational workflow is adaptable for a broad spectrum of drug candidates, from early-stage to late-stage, shelved, marketed, or generic drugs, assisting in defining indications, combinations, or targeted doses and identifying responsive patients across different cancer indications.
The FDA recently cleared the Investigational New Drug (IND) application for Lantern's internally developed LP-184, a synthetically-lethal small molecule expected to enter Phase 1A clinical trials this summer for advanced solid tumors and brain and CNS cancers. The AI drug discovery company marked a key milestone in March 2023 by dosing the first patient in its multi-center Harmonic™ Phase 2 trial, aimed at transforming therapeutic strategies for underserved populations such as never-smokers diagnosed with advanced non-small cell lung cancer (NSCLC).
In November 2022, Lantern Pharma also presented promising preclinical results for LP-184 at the Society for Neuro-Oncology (SNO) annual meeting. LP-184 targets glioblastoma (GBM) cells by damaging their DNA and showed enhanced efficacy when combined with FDA-approved agent spironolactone. Preclinical models indicated that LP-184 was 5,000 times more potent than current GBM therapy, Temozolomide. The FDA has awarded LP-184 Orphan Drug Designation for several cancers, including malignant gliomas, atypical teratoid rhabdoid tumors (ATRT), and pancreatic cancer, along with a Rare Pediatric Disease for ATRT.
Lantern's portfolio now includes four drug candidates across two Phase 2 programs, an antibody-drug conjugate program across 12 cancer indications, and several partner programs, illustrating the platform's potential in enhancing therapeutic pipelines in the industry.
Integrating AI into CNS drug discovery projects
Lantern Pharma, harnessing the power of its RADR® platform, has emerged as an innovative player in the complex field of CNS drug discovery, particularly around the challenge of the blood-brain barrier. The company is open to partnering with entities that have extensive molecular databases spanning not only neuro-oncology, but also Alzheimer's, and other cognitive disorders. Their AI algorithms enable high-speed screening of thousands of molecules on critical parameters such as BBB permeability. This approach can save substantial time and resources, potentially accelerating the drug discovery timeline, and promising transformative changes in treating CNS disorders.
Topics: NeuroTech