Cracking a Drug Delivery Puzzle
Drug delivery technologies often become a remedy and a solution for various drugs on their way to clinical approval. When the drug is developed, whether it is a small molecule, peptide, nucleic acid, antibody, or even the whole living cell, it is essential to address the possible delivery hurdles and choose the proper strategy to target the right site with the right drug amount.
Knowing the challenges and needs of various drug classes shapes several delivery paradigms for improving a drug’s therapeutic function. The Nature review paper classified the delivery paradigms into three classes: modification of the drug itself; alteration of the environment around the drug; and creation of an interface between the drug and its surrounding. In the last case, such an interface is a drug delivery system, that facilitates delivery by controlling the interactions between the drug and its microenvironment.
Nanoparticles are an example of a drug delivery system, the third described paradigm. In our recent article Current Trends in Nanomedicine: Notable FDA Approvals and Clinical Trials to Follow we discussed three main classes of nanoparticles and the success stories behind their application in drug development.
In brief, lipid-based nanoparticles and liposomes offer high biocompatibility but restricted drug load; simultaneously, polymer-based nanoparticles have a set of advantages over their counterparts but still tend to aggregate and provoke toxicity effects. Nanospheres, nanoshells, nanostars, and nanocages -- this all applies to inorganic nanoparticles, with multiple examples of FDA-approved drugs. However, the toxicity and solubility limitations prevent inorganic nanoparticles from becoming the universal solution.
Regardless of nanoparticles being a significant and diverse class of delivery systems, there are more examples of innovative solutions in the field, such as microneedle patches, swellable hydrogels, polymer films, wound dressing, and more.
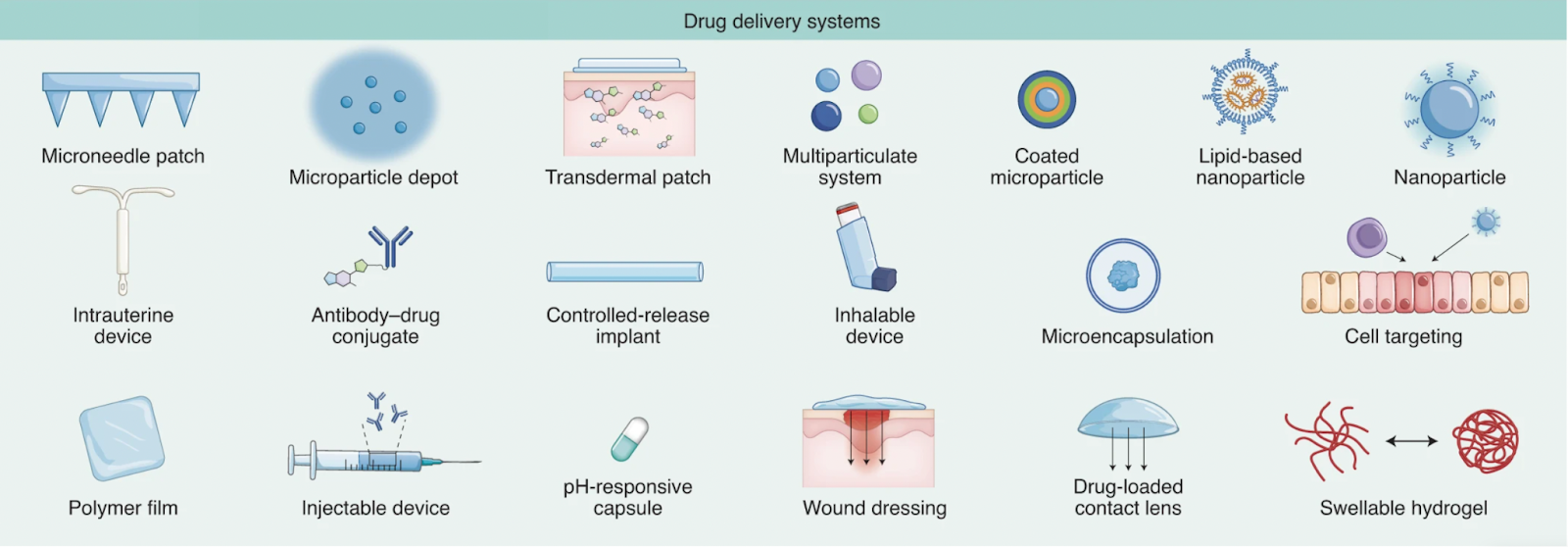
Drug delivery systems. Credits: Vargason et al., 2021 https://doi.org/10.1038/s41551-021-00698-w
Notably, the vaccine industry could largely benefit from such a drug delivery system as microneedle patches. It is not only convenient and painless for the user but also provides functional advantages such as the prolonged exposure of the human body to the antigen, giving a sufficient time frame to the immune system. Lastly, microneedle patches can be stored and delivered at room temperature, with no need for refrigerators and freezers.
Various materials can be used for microneedle technology, from metal to polymer, glass, and ceramic, as well as water-soluble or biodegradable materials. The used material will directly influence the drug delivery properties of the patch.
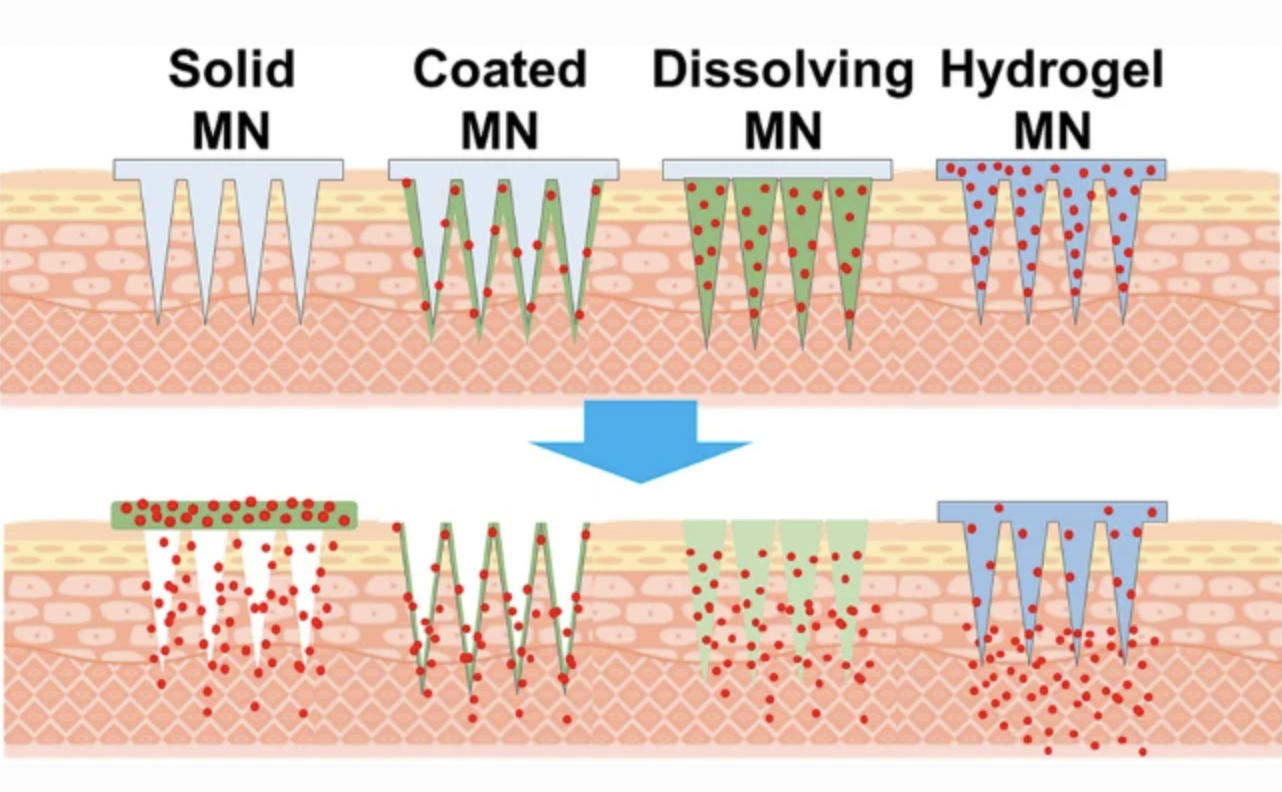
Schematic illustration of the microneedle types and their drug delivery properties. Credits: Jung, J.H & Jin, S.G., 2021 https://doi.org/10.1007/s40005-021-00512-4
Cambridge-based Vaxess Technologies is a biotech company developing vaccine patches using biomaterials derived from silk protein. Their proprietary MIMIX patch technology promises compatibility with a broad array of vaccines and therapeutics, ensuring a sustained release of treatments via the skin over the desired period. Their microneedle patch loaded with influenza vaccine is currently going through phase 1 of clinical trials.
INTERVIEW: Ancient Material Promises To Solve Drug Delivery “Pain”
As with any emergent field, microneedle technology attracts acquisitions and investment deals. In October 2022, Emergex Vaccines Holding Limited acquired Zosano Pharma Corporation, which developed a proprietary microneedle array patch and manufacturing equipment for it. The microneedle technology would complement current Emergex’s developments with fully synthetic CD8+ T-cell adaptive vaccines.
At the same time, microneedle patches can find application in relatively unexpected directions, such as a peanut butter allergy. The study carried out at the University of Michigan demonstrated that the peanut-allergic mice wearing a microneedle patch for 5 minutes a week turned fully desensitized in slightly more than a month. Such a technological approach could ease the pain and demolish the danger of multiple food allergies just by inserting the allergens into the upper layers of the skin.
Back to the nanoparticles topic, well-known RNA therapeutics would hardly be able to exhibit any therapeutical function without the proper drug delivery technology support. To act as a drug, first, RNAs should get into the cytosol of a living cell, which is a challenge in itself since foreign nucleic and ribonucleic acids are not supposed to disrupt and cross the cell barrier.
This is where multiple companies try to use nanoparticles as a vehicle for mRNA intracellular delivery. AstraZeneca concentrates its efforts on lipid nanoparticle technology for mRNA delivery so that it can be translated into the protein inside the cells after intravenous, subcutaneous, or lung administration. At the same time, nanoparticles can also assist with targeted antibody delivery for immunotherapy purposes. In collaboration with Memorial Sloan Kettering and Cornell University, AstraZeneca demonstrated that nanoparticles containing engineered antibody fragments penetrated the HER2-overexpressing breast cancer tumor and accumulated there.
In October 2022, SiSaf Ltd, an RNA delivery and therapeutics company, announced that it is initiating the FDA Regulatory process to obtain an Orphan Drug Designation for their siRNA therapeutic for patients with a rare genetic skeletal disorder. The drug utilizes SiSaf’s silicon-stabilized hybrid lipid nanoparticles (sshLNP) technology named Bio-Courier, which merges lipid nanoparticles with inorganic bioabsorbable silicon. Using such nanocarriers enables the intracellular delivery of siRNA to the target site, suppressing the expression of the mutant gene.
Innovative drug delivery systems also demonstrate promising results for glaucoma patients. In May 2022, Nature Communications’ paper described the intelligent theranostic contact lens for electrical sensing and regulation of intraocular pressure, delivering anti-glaucoma drugs on demand. The drug delivery modulus employs a wireless power transfer circuit to trigger the delivery of the drug into an aqueous chamber via iontophoresis. This technology is yet to be tested clinically, but such a smart contact lens could potentially solve the unmet need for glaucoma patients in the future.
Also, in March 2022 FDA approved Johnson & Johnson’s contact lens loaded with the antihistamine ketotifen, aiming to reduce the itchiness and other signs of allergic reaction of people wearing lenses. This approval just confirmed one more time that the idea of futuristic lenses which release drugs is now a reality and hopefully can work for several other pathological conditions.
