The Rise of Synthetic Biology Platforms
Even though the term ‘synthetic biology’ remains foreign to an average person, the idea behind it is familiar to many. Nearly 3 decades ago Steven Spielberg released a famous sci-fi movie where a bunch of genetically modified creatures escapes the premises and starts terrorizing human beings. And while Hollywood scenarists use stories similar to this one from ‘Jurassic world’ to play on the audience’s nerves, major scientific advances in life sciences over the last 50 years provided researchers with technologies that could turn those scenarios into reality.
So what is synthetic biology? Polish geneticist Waclaw Szybalski introduced this term to designate the engineering of natural biologic systems such as bacteria, yeasts and fungi, plants and animals to produce the desired products. A known example of synthetic biology application is rice modified to produce beta-carotene, a nutrient usually associated with carrots, to prevent child vitamin A deficiency in poor countries.
There are two main ways in which synbio strategies are implemented nowadays:
-
Engineering biosynthetic pathways, gene networks, proteins, and molecular switches for optimizing or enhancing natural cellular functions for in vitro or in vivo applications.
-
Engineering naturally-existing bacterial, viral, plant and mammalian cells or constructing new cells or organisms de novo for obtaining novel functional outputs with therapeutic applications.
First generation of synthetic biology companies emerged in the early 2000s. Back at the time, scientists were exploring the possibility of reading the genetic code responsible for producing specific molecules fast and at a low resource investment through breakthroughs in DNA sequencing and solid-state spectroscopy. Researchers would aim to characterize and identify needed parts of DNA sequences corresponding to the functions of interest, combine the parts into devices to achieve more complex functions or enhance existing ones, then insert novel machinery into cells. However, with the development of science the complexity and unpredictability of this machinery increased, and their in vitro design was complicated by lack of characterization of the DNA sequences and limited engineering and designing capabilities. Synthetic biology required reliability, standardization, and automation of the design.
Evolva and Amyris are typical representatives of synbio firms of 2000-2010. These companies focused on a singular product line - biofuels, aspiring to find a more sustainable analogue to oil. As promising as the idea sounds, these companies faced some issues when they tried to scale up and produce cost-competitive fuels. Designing the DNA sequence, engineering the organisms, fermentation, manufacturing, and downstream processing - all these processes appeared to be overwhelmingly complex when businesses attempted to do it all by themselves.
As a response to this challenge, in the following decade of 2010s companies with platform technologies offering ‘synthetic biology as a service’ emerged on the market. Among them one could find Ginkgo Bioworks, Zymergen, and Twist Bioscience offering outsourcing services of creation of engineered organisms and nucleic acids for various applications. Some companies such as Synthace and Teselagen Biotechnology built AI-powered software to efficiently design DNA/RNA sequences.
Division of research processes brought remarkable progress to the field - computer-aided design, synthesis, and testing of DNA/RNA and engineered organisms using technologies such as CRISPR, pooled oligo synthesis, NGS, and training ML algorithms to accelerate the designing process. These advances resulted in drastic cost reduction and fastening the sequence design, leading to a significant increase in the number of synthetic genomes.
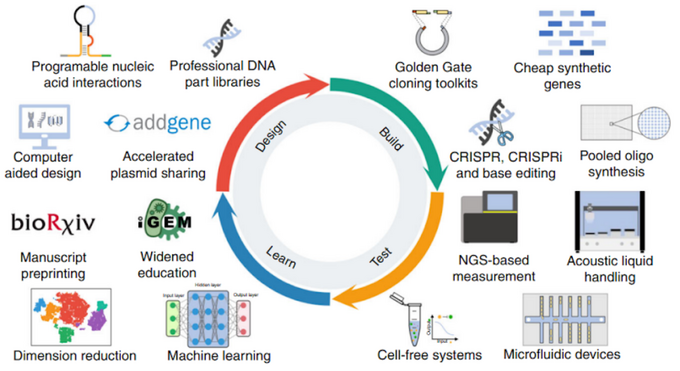
In the third decade of the 21st century synbio market has seen a threefold increase in the number of the represented companies offering services in diverse fields, from agriculture to drug design. The aforementioned companies such as Ginkgo Bioworks and Zymergen are transforming the field by developing an ecosystem of partners and customers, turning them into ecosystem engineers. For instance, Zymergen has partnered with Sumitomo Chemical to develop a biopolymer film called ‘Hyaline’.
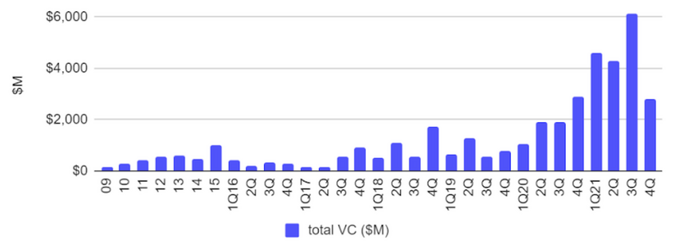
Amount of investments in synthetic biology over the 2009 - 2021 period
With regard to its rapid development and innovative solutions there is no wonder that synthetic biology caught the attention of multiple investors. Tech leaders of the last century including Bill Gates, Peter Theil, Marc Andressen, Eric Schmidt, Vinod Khosla, and many others who have led investments into major synthetic biology companies such as Zymergen, Ginkgo Bioworks, Twist Bioscience through their VC funds (Founders Fund, Andressen Horowitz, Lux Capital, General Catalyst, etc.). As a result, the amount of investments in the field rose from $100 million in 2010 to $18 billion in 2021.

