Lantern Pharma Completes Japan Enrollment in AI-Guided Lung Cancer Study, Reports Durable Response Case
Lantern Pharma has completed patient enrollment in Japan for the Phase 2 HARMONIC trial of a disulfide small molecule LP-300 in never-smokers with non-small cell lung cancer (NSCLC). Ten patients were enrolled ahead of schedule at five clinical sites, including the National Cancer Center in Tokyo. This marks progress in the trial’s global expansion, which targets around 90 participants across the U.S., Japan, and Taiwan—regions with higher prevalence of never-smoker NSCLC. According to Lantern, never-smokers account for 33–40% of NSCLC cases in Japan, compared to 15–20% in the U.S.
The company, based in Dallas, develops oncology therapeutics using its RADR platform, which leverages over 100 billion oncology-specific data points and machine learning to predict treatment responses and uncover viable drug candidates.
HARMONIC is a multicenter, open-label, randomized Phase 2 trial evaluating overall and progression-free survival in never-smoker patients with relapsed advanced NSCLC following tyrosine kinase inhibitor (TKI) therapy. The trial compares standard chemotherapy (carboplatin/pemetrexed) alone versus in combination with the investigational drug LP-300, which was approved by the U.S. FDA for investigational use in this study. LP-300 is designed to work alongside chemotherapy by targeting tyrosine kinase gene pathways implicated in tumor growth and spread. NSCLC in never-smokers often involves mutations in TK genes such as EGFR, ALK, ROS, and MET, which drive oncogenesis.
In addition to never-smokers, former smokers with NSCLC exhibiting similar genetic profiles may also be eligible to participate. Lantern’s RADR (Response Algorithm for Drug Positioning & Rescue) platform was used to advance LP-300 by confirming its mechanism of action and identifying combination synergies. RADR integrates oncology datasets and machine learning tools to predict drug response, drawing from public databases, commercial clinical trials, and proprietary 3D tumor models.
See also: How AI Enables Precision Oncology
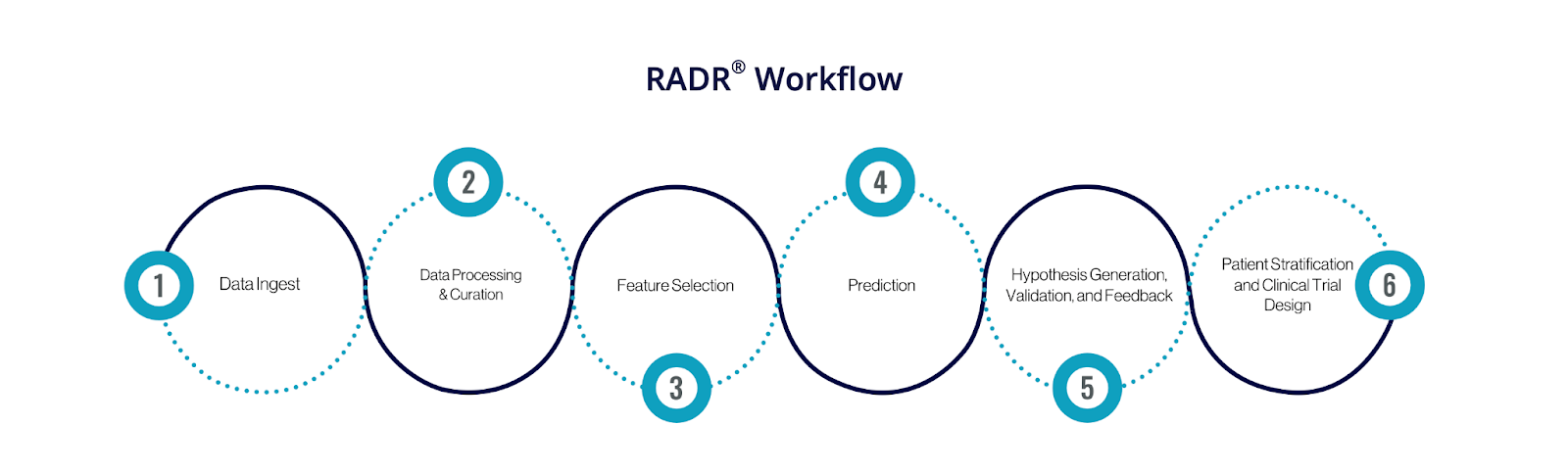
Image credit: Lantern Pharma. The company’s AI-driven drug discovery platform RADR workflow
In a previously reported lead-in cohort of seven U.S. patients, the trial showed an 86% clinical benefit rate and 43% objective response rate. One patient—a 70-year-old never-smoker with advanced NSCLC—achieved a complete response in lung and adrenal lesions and has sustained that response for nearly two years across 21 treatment cycles, with no dose-limiting toxicities or significant adverse events reported.
Lantern plans to release additional trial data from both U.S. and Asian cohorts by the end of Q3 2025. The company estimates the market for treating never-smoker NSCLC at over $4 billion annually. Currently, there are no therapies approved specifically for this subset of lung cancer patients.
Topics: Clinical Trials