Will Biologics Surpass Small Molecules In The Pharmaceutical Race?
The first biologics drug, humanized insulin (5.8 kDa), became available in 1982 following the advent of biotechnology, and it marked a new era in the pharmaceutical industry. Modern advances in biotechnology enabled large-scale syntheses of biologics in a more or less cost-effective manner. Having once started with large peptides and recombinant proteins, biologics nowadays include a wide range of other entities, such as monoclonal antibodies, nanobodies and related objects, soluble receptors, recombinant DNA, antibody-drug conjugates (ADCs), fusion proteins, immunotherapeutics, and synthetic vaccines.
RELATED: The Explosion of Therapeutic Modalities: Small Molecules, Biologics, and Everything in Between
The growing promise of biologics drugs has captured the increasing attention of pharmaceutical professionals and industry analysts, as this alternative drug discovery paradigm represents substantial business challenge for a more "traditional" small molecule-oriented research model, dominating the pharmaceutical industry for more than a century.
So, here is the question: are small molecules losing “attractiveness” as research objects for drug discovery in the modern world of biological advances? Some experts remain optimistic about the potential of small molecules to lead the race in the future pharmaceutical pipelines, others perceive that “money are changing pockets”, and small molecules research will be increasingly neglected by shareholders and investors in favor of biologics and therapies.
In order to figure out where the industry is heading, let’s first start with a bit of statistics. Over a period of 10 years (2010-2020) U.S. Food and Drug Administration (FDA) approved a total of 421 new molecular entities (NMEs), excluding several diagnostic imaging agents and 1 insulin analog. It appears that 76% of them are small molecules (321) with only a quarter being biologics, with a quite stable distribution ratio year by year since 2017. Interestingly, there is no dramatic growth in biologics NMEs approvals relative to small molecules as both categories of therapeutics are trending in a quite similar fashion, as shown on the diagram below. However, since the biologics are priced very differently from small molecules (they are way more expensive), the picture is different when looking at total sales and revenue growth: for instance, over the period from 2011 to 2017 biologic sales revenue has grown by 70% having reached $232 billion. The share of the total pharmaceutical market that biologics held in that period increased from 16% in 2006 up to 25% in 2016, and according to EvaluatePharma report for top 20 product sales in 2020, biologics had a small edge over small molecules, with biologics accounting for 12 products (60%) and small molecules -- 8 products, respectively (40%). In today's drug market, small molecules constitute as much as 90% of global sales. However, in the United States and other countries, the rate of purchase of biologics is rapidly increasing, by those who can afford them. On average, a daily dose of biologic costs 22 times more than that of a small molecule.
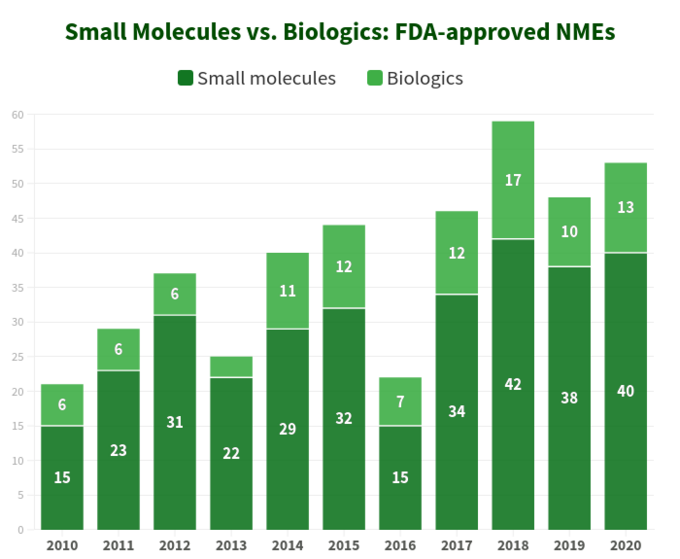
While there are distinct advantages of biologics over small molecules in several ways (for example, their profound selectivity), things are not “black-and-white” when comparing these two categories of therapeutics by their attributes, as both of them have substantial pros and cons to consider during strategic decision-making.
A table below summarizes some strong and weak aspects of both categories (ref) illustrating challenges and opportunities available in both “camps” (note colors: gray -- no apparent advantage in neither camp; green -- advantageous situation; yellow -- disadvantageous situation):
Small Molecules |
Biologics |
|---|---|
|
General properties |
|
|
Low molecular weights (0,1 - 1 kDa); usually chemically and thermally stable, wide range of polarity. |
Very large molecular weights >1 kDa; generally polar, sensitive to heat, easily degraded (with the exception of some long-lived types such as monoclonal antibodies) |
|
Selectivity, safety |
|
|
Rather promiscuous, usually bind to various off-target sites, rendering side-effects or toxicity. Finally, biologic developers have had an easier time obtaining patents because there is was little or no state-of-art in the field, when current blockbusters were discovered. |
Highly specific to the targets, generally of lower toxicity (with one major exception being that of immunogenicity, which may seriously influence efficiency, safety, and disposition of biologics). |
|
Cell permeability |
|
|
SMs bind with targets like G-protein-coupled receptors (GPCRs), ligand-gated ion channels, and receptor tyrosine kinases on the extracellular or intracellular domains. They can access targets in the intracellular regions, cytosols, nuclei and even CNS targets, separated by the tight blood-brain barrier (BBB). |
A large proportion of pharmacological targets are embedded and therefore inaccessible to biologics. Especially, when it comes to the central nervous system (CNS), the presence of the blood-brain barrier is a major obstacle on the way of any molecules larger than 600 Da restricting up to 98% of SMs and practically all biologics. |
|
Delivery |
|
|
Largely fall into “Rule of five” for oral absorption, making it suitable for oral delivery. Further permeability via intestinal epithelium is primarily mediated by a combination of passive diffusion and paracellular transport. |
Intrinsic instability and high molecular masses render nearly all biologics orally inactive. Mostly, invasive delivery, or alternative non-invasive technologies are in progress. |
|
Distribution |
|
|
SMs are distributed via the blood circulation, allowing for achieving pick concentrations quickly. |
For larger molecules (e.g. >10 kDa) a slower (by 100–500 times) lymphatic system becomes dominating in the distribution process. Larger biologics distribute via both the blood and the lymphatic systems moving convective transport, receptor-mediated endocytosis, phagocytosis, and pinocytosis. It The result of this situation is that larger biologics have longer half-lives, limited volumes of distribution, and need more time to reach peak concentrations, compared to SMs. |
|
Disposition (metabolism) |
|
|
Most SMs are disposed of by non-targeted organs -- via cytochrome or non-cytochrome metabolisms, renal filtration, or fecal excretion. |
Biologics have tighter interactions with targets, so their dispositions are directly affected by their binding (receptor-mediated drug disposition), including the clearances of biologics by proteases and peptidase. |
|
Drug-drug interactions |
|
|
SMs are prone to drug–drug interactions that can occur due to the presence of concomitant drugs that affect their transport, metabolism, transport, or elimination pathways. |
Biologics are less prone to traditional drug–drug interactions since they undergo metabolism and elimination as the endogenous substrates. However, there are documented cytokine-mediated changes in drug-metabolizing enzymes, therefore drug-biologic interactions have to be assessed in the case the drug might influence the expression of metabolic enzymes. |
|
Business aspects |
|
|
Low prices for SM drugs. |
Typically, very high prices for biologics treatments. This is regarded as one of the reasons biologics appear to be delivering better overall economic returns, compared to SMs. |
|
High rate of attrition (2009 study by KMR Group showed that only 7.1% of SMs entering preclinical testing eventually reached the market). |
Relatively low rate of attrition (same KMR Group study revealed 24.4% of preclinical stage biologics survived up to the market stage). |
|
Severe competition from chemical generics after patents expiration. |
Biologics developers face less severe competition from biosimilars, after patents expiration. |
|
Simpler drug discovery/development process. |
More expensive and complex drug discovery and development process, compared to biologics. |
Considering the above comparison, it becomes obvious that biologics are not a “magic bullet” and the industry will not be dominated by biologics in the foreseeable future, rather, a competitive equilibrium will be maintained between small molecules, biologics, and hybrid forms of therapeutics, such as ADCs -- with local domination of each form in more suitable therapeutic areas, use cases, etc.
Furthermore, below is a list of several industry drivers which are playing on the side of small molecules and might influence the balance of powers in the total pharmaceutical market in favor of chemical starting points:
1. The rise of artificial intelligence (AI) in drug discovery
Following breakthroughs in deep learning algorithms (2012), the rise of generative adversarial networks (GANs), able to excel in a number of research tasks, the interest in various AI technologies has skyrocketed in pretty much every industry. Many AI-powered tools have quickly become commercial mainstream, like chatbots, personal assistants, autopilots, etc.-- which is real-world proof of concept of AI feasibility.
Drug discovery is not an exception to this “AI-driven trend”, and the number of startups trying to apply AI to boost drug discovery in different ways has grown substantially over just several years, having reached over 350 active companies, including early-stage startups as well as more developed companies and IPO stage companies (according to an interactive report "The Landscape of Artificial Intelligence (AI) In Pharmaceutical R&D"). There has been a wave of breakthroughs in this area recently, where AI systems helped rapidly discover and develop first-in-class small molecules, and those entered clinical studies -- by companies such companies as Insilico Medicine, Exscientia, BenevolentAI, Recursion Pharmaceuticals, Deep Genomics, and others.
Interestingly, the majority of AI-driven startups are focused on small molecules drug discovery, rather than biologics, which is, probably, not surprising. Historically, even non-AI computational methods (cheminformatics) were primarily used for small molecule therapeutics due to their substantially simpler molecular structures and interaction patterns.
The below diagram shows estimated statistics for 319 actively marketed drug discovery startups developing or applying specialized AI-tools in their research workflow. As you can see, a half of all the companies (49%, 156 startups) are focused on small molecules, while only 20% (64 startups) are involved in discovering/developing biologics drugs (antibodies, vaccines etc). The disproportion towards small molecules is also well illustrated by the amount of VC funding raised collectively by AI-driven startups and scale-ups since 2011 (according to data available in the BiopharmaTrend report)
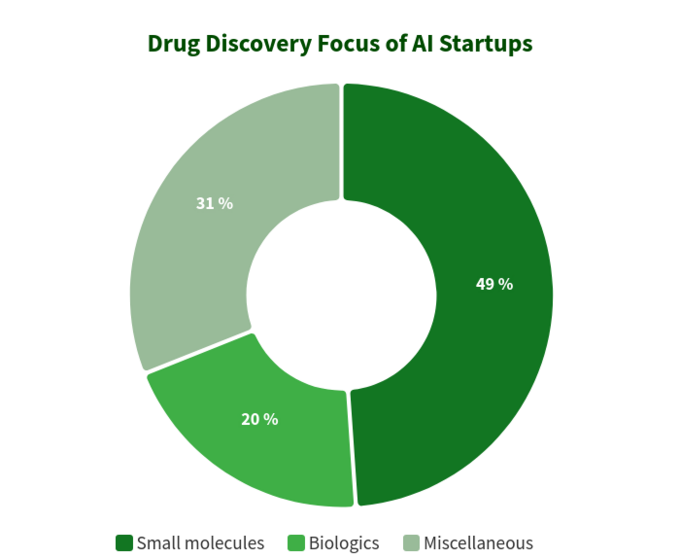
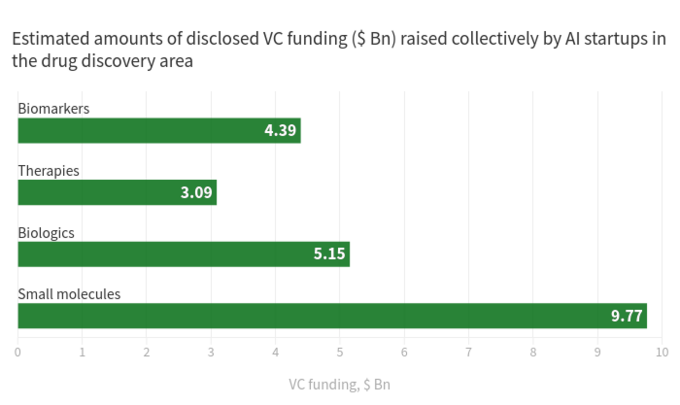
This situation suggests that recent advances in AI algorithms and a trend to apply machine learning for early stage pharmaceutical research tend to be driving more growth in small molecule drug discovery, as compared to biologics discovery -- at least for now. This might lead to more future investments in small molecule-focused projects backed by AI-driven technologies.
2. Reaching “undruggable” targets
Such important targets as, for example, protein-protein interactions (PPIs), are traditionally dominated by biologics (mAbs) as potential actors, while small molecules had long been considered unsuitable in this case due to their small size.
With the advances in pharmacogenomics, this situation can change rather quickly following the approvals maraviroc (514 Da) and tirofiban (441 Da). It was revealed computationally that SMs can actually have relatively high affinities to specific interfaces of proteins and, importantly, they can modulate the ‘intrinsically disordered protein regions’ linked to a set of complex systems diseases. Here is one interesting cover up of this topic summarizing some of the important insights into PPI machinery obtained over recent decades.
Another rapidly growing area for small molecule drug discovery is targeting ribonucleic acid (RNA). This topic was listed in “R&D Trends To Follow In Pharmaceutical Industry In 2021 And Beyond”, and also there was a detailed review of the topic in C&EN recently.
Another fresh idea to unleash the power of small molecules is the emerging and quite disruptive area of small molecule drug discovery -- targeted protein degraders (TPDs). A lot has been written about TPDs, for instance, here is a great review of the topic, published in Nature. Recently, I also interviewed Dr. Alessio Ciulli, Professor and Director of Centre for Targeted Protein Degradation (CeTPD) at the University of Dundee, and Dr. Danette Daniels, Group Leader at Promega Corporation about the promise of TPD modality. The strategy here is to develop bifunctional small molecules able to route a targeted protein altogether to the proteasome, the cell’s trash-removal machine, instead of just inhibiting the protein’s action as in a ‘traditional’ approach. Notably, typical protein degraders are usually substantially beyond the "Rule of 5".
3. Overcoming limitations of screening technologies
A lot of research potential of small molecules has not been yet realized due to various technical limitations of screening approaches used for identifying starting points in drug discovery programs.
One such example is phenotypic screening approach, which is experiencing a renaissance nowadays due to several technological advances, including the increasing ability to develop physiologically relevant cell models, readouts, and sophisticated detection technologies helping reveal mechanisms of action (MoA) more efficiently, minimizing uncertainty. On the other hand, the implementation of large-scale profiling techniques and computational methods, using deep learning, offers a new level of systems analysis and understanding of small-molecule phenotypes.
Another promising screening paradigm is via using DNA-encoded libraries (DELs). Due to its unique arrangement, DEL technology provides a suitable way of testing hundreds of millions and even billions of novel molecules in target-based drug discovery programs. While this technology is not without challenges (e.g. limitations of the DNA-compatible chemistry, uncertainty with screening hits, etc), the area is growing fast and is even regarded as ‘revolutionary’ for small molecule drug discovery.
4. Overcoming the synthesizability barrier
One of the stumbling stones of early-stage small molecule drug discovery is a limitation of synthetically accessible chemical space -- while a lot of promising chemical ideas can be generated computationally, or otherwise, there is always a risk that synthesizing the whole set of compounds to validate such ideas would be costly, or even unfeasible.
Recent advancements in this area have been made, again, using AI-based software offering human-level synthesis planning performance. A more empirical strategy was developed by a chemical producer Enamine, which is known as REAL Chemical Space, and currently includes around 23 billion make-on-demand molecules for hit exploration and other medicinal chemistry tasks. The concept boils down to applying a set of in-house validated chemical routes (over 170 reactions) to a large pool of chemical building blocks available in Enamine's own stock (above 112 thousand compounds) -- with the aim of combining them in a combinatorial fashion to produce a cascade of larger “lead-like” or “drug-like” molecules. Once a certain degree of reproducibility and yield is achieved in a large enough set of experiments (at least 80%), a further computational enumeration takes place to build up a wider chemical space based on the experimentally validated cases.
New R&D markets and roles for small molecules
All in all, it is hard to underestimate the size and growth potential of small molecules pipelines. It is also important to note that small molecule drugs could play an important role in some next-generation medicines, making them reliant on small molecule pipelines. For example, in the case of stem cell therapy, small molecules can trigger therapeutic reactions.
As was highlighted during CPhI North America by MilliporeSigma’s Jeffrey Shumway, small molecule products are tending to become more complex, altering their role in the (bio)pharmaceutical industry.
Finally, the future area for small molecules is antibody-drug conjugates and related objects, which emerged as a promising class of therapeutics due to the natural convergence of two drug discovery paradigms and multiple technological trends.
Finally, there is a whole world of therapeutic modalities taking best from both worlds, for example, small-peptide drugs, which may in some aspects unite the good of both worlds - favorable delivery and cost and targeting undruggable surfaces (PPI).
Disclaimer: The information is for general awareness only, and is not legal/financial/stock trading/medical advice of any kind. You read it at your own will and any use of this information is at your own risk. It is your responsibility to evaluate the usefulness and safety of the information in this post, and the Internet generally, and how it applies to your own situation and then consult the relevant experts for professional advice if required.