Cross-Coupling Hits The Big Time Again
Recently, a series of papers emerged in scientific press significantly expanding capabilities of classical carbon-heteroatom cross-coupling reactions making them simpler, cheaper, and efficient across a much broader range of substrates.
Cross-coupling reactions are widely used by medicinal chemists since they offer a suitable way of creating new carbon-carbon, carbon-oxygen, carbon-sulfur and carbon-nitrogen bonds, which are the staple of modern drug development. Despite tremendous progress in optimizing C-C bond-forming reactions (most notably, Negishi, Suzuki–Miyaura, Stille, Kumada and Hiyama couplings), the use of carbon-heteroatom couplings was rather limited in practice. Fortunately, a major progress has been made recently in this direction.
Photoredox catalyst + Ni-catalyst = new capabilities
C-O coupling
Last year, an article published in Nature by a group of scientists from Princeton University - Jack Terrett, James Cuthbertson, Valerie Shurtleff and David MacMillan, - introduced a new highly efficient and universal carbon-oxygen (C-O) coupling reaction using abundant alcohols and aryl bromides, activated by a combined effect of photoredox and nickel-bearing catalysts.
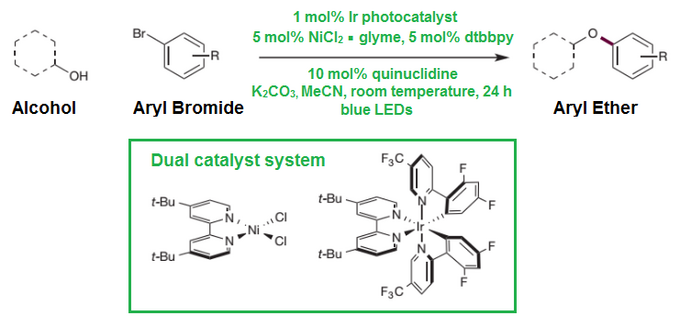
More importantly, authors suggested a new general strategy to activate important organometallic mechanisms by means of modulating oxidation state using only weak light and single-electron-transfer catalysts.
C-S coupling
The beginning of this year was marked by the two prominent papers both introducing novel routes for C-S bond formation (thioetherification) using similar photoredox-nickel dual catalysis approach as mentioned earlier in 2015.
The paper published in Organic Letters by scientists from The University of Pennsylvania - Matthieu Jouffroy, Christopher Kelly, and a group leader Prof. Gary Molander, reports on a base-free, room temperature, S-selective method for the thioetherifcation of aryl bromides, tolerating a variety of aryl and heteroaryl bromides. The reaction could be potentially suitable to prepare sulfides with biological relevance or for bioconjugation. Moreover, it provides unprecedented access to new chemical space for thioethers - a promising area for examination by drug discovery groups and agriscience.

In the other paper published in JACS, authors from AstraZeneca Pharmaceuticals - Martins Sunday Oderinde, Daniel Robbins, Brian Aquila, and Jeffrey Johannes, together with their colleague Mathieu Frenette from Université du Québec à Montréal, present a new, robust, mild and highly chemoselective method for the cross-coupling of aryl, benzyl and alkyl thiols with a wide array of functionalized aryl and heteroaryl iodides, induced by light in presence of Ni-catalyst. The authors are the first who found an efficient method allowing iodoarenes to engage in transition-metal catalyzed cross-coupling reactions with thiyl radicals or any heteroatom radical. This catalytic system becomes especially suitable in practice, as it operates with high efficiency in the presence of molecular oxygen.
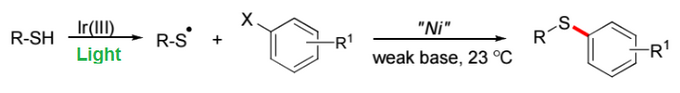
Both works are of great interest for medicinal chemistry as C-S bond is peculiar to many drug molecules across various disease types such as cancer, HIV, and Alzheimer’s disease.
C-N coupling
Finally, a fresh paper published this June in Science reports on the application of photoredox catalysis for conducting aryl amination in the presence of ligand-free Ni(II) salts, which is a cheap and direct complementary method for classical C-N coupling reaction.

This time, a success was achieved by joint efforts of researchers from Merck Center for Catalysis at Princeton University - Emily Corcoran and David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry, their colleagues from Merck Research Laboratories - Shishi Lin, Spencer Dreher, Daniel DiRocco, Ian Davies, together with researchers from MIT - Michael T. Pirnot and a group leader Stephen Buchwald, the Camille Dreyfus Professor of Chemistry, whose works in the area of C-N bond couplings are well known as Buchwald-Hartwig aminations.
The authors reported that the reaction only needed tiny amounts of photoredox catalyst in combination with a very cheap and simple nickel catalyst to give substantial yields for a range of coupling partners.
The reaction mechanism for the above dual catalyst systems was suggested by Mark Levin, Suhong Kim, and F. Dean Toste from University of California in their paper “Photoredox Catalysis Unlocks Single-Electron Elementary Steps in Transition Metal Catalyzed Cross-Coupling”, published in May.
From lab to industry
The new photoredox C-N coupling method was finally tested in Merck & Co on a so-called 'informer' library that has been used to benchmark other methods. It was found that 78% of the drug-like compounds in the library underwent successful coupling, suggesting that the newly developed protocol would find broad applicability in medicinal chemistry.
An attractive feature of the reaction is that a mild base can be used to run it, which is helpful for performing chemistry on sensitive substrates. Moreover, the homogeneity of the reaction makes it especially suitable to flow conditions, in which the reaction solution is steadily pumped through a reactor.
Topics: Tools & Methods
