Lantern Pharma Gets FDA Green Light for AI-Guided Trial Targeting Hard-to-Treat Lung Cancer
The company’s second Phase 1b/2 trial of LP-184 within a month will test the AI-developed small molecule in patients with lung tumors that resist standard treatments.
Lantern Pharma has received FDA clearance to begin a Phase 1b/2 trial of LP-184 in non-small cell lung cancer (NSCLC) patients whose tumors carry KEAP1 and/or STK11 mutations and express low levels of PD-L1—a subgroup with poor response to chemo-immunotherapy and median survival around 15 months. The study will evaluate LP-184 in combination with checkpoint inhibitors nivolumab and ipilimumab.
See also: How AI Enables Precision Oncology
This follows last week’s clearance for a separate Phase 1b/2 trial of LP-184 in triple negative breast cancer (TNBC), where the drug will be tested alone and with the PARP inhibitor olaparib. Both programs stem from Lantern’s AI platform, which identifies cancer types likely to respond based on tumor-specific expression of PTGR1—an enzyme that activates LP-184 inside cancer cells but not healthy ones.
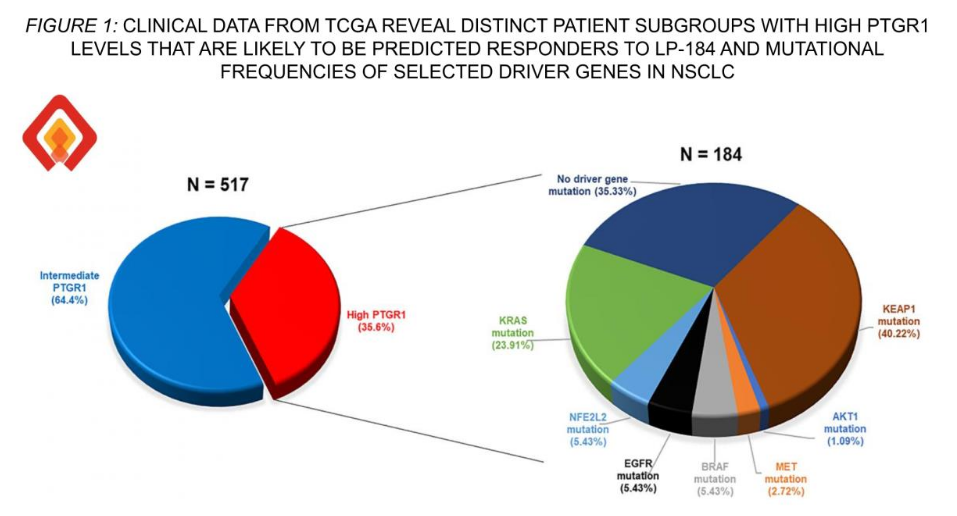
Refining patient stratification for LP-184—Lantern Pharma leverages large-scale clinical datasets to pinpoint genomically defined NSCLC subsets most likely to benefit from LP-184-based regimens, aligning therapeutic mechanism with tumor biology to guide precision trial design.
In NSCLC, about 35% of tumors show high PTGR1 levels, and among those, 40% also carry KEAP1 mutations, making them strong candidates for LP-184. The company plans to seek Fast Track or Accelerated Approval based on trial outcomes. Lantern estimates this lung cancer subgroup represents a $2 billion annual market opportunity.