A Running List Of Covid-19 Treatments In Development (Not Updated)
Disclaimer: This post is not medical advice, it is only for informational purposes. In case of need, consult your doctor at all times to make decisions about your health.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a positive sense, single-stranded RNA beta coronavirus, a member of Beta-CoV lineage B (subgenus Sarbecovirus).
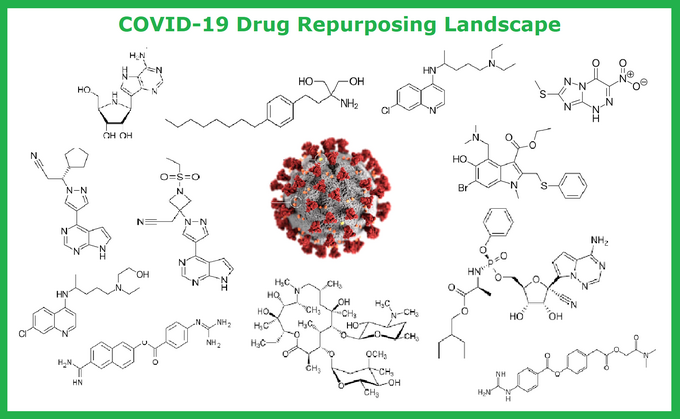
COVID-19 represents a global health threat and it is a serious driver of possible economic crisis, so curbing the current ongoing outbreak is the matter of top priority for most health organizations and biotech companies.
Topics: Novel Therapeutics
Comments