Ultromics Gets $55M to Spot Hidden Heart Failure with AI Ultrasound
Oxford-based Ultromics has secured $55 million in Series C funding to expand deployment of its AI-powered echocardiography software across the U.S. and globally. The company develops FDA-cleared tools that analyze routine ultrasound data to detect subtle signs of heart failure, including hard-to-diagnose conditions like HFpEF (heart failure with preserved ejection fraction), a form of heart failure where the heart pumps normally but is too stiff to fill properly, and cardiac amyloidosis, a condition where abnormal proteins build up in the heart muscle and interfere with its function.
Ultromics’ AI system is designed to integrate into existing clinical workflows without added hardware, aiming to identify high-risk patients earlier using standard echocardiogram data.
Founded in 2017 as a spinout from the University of Oxford by Dr. Ross Upton and Prof. Paul Leeson, Ultromics has now raised about $100 million across multiple rounds. Its Series A in 2018 brought in £10 million from Oxford Sciences Innovation (OSI), Neptune, RT Ventures, GT Healthcare, and others. A $33 million Series B in 2021, led by Blue Venture Fund with participation from Optum Ventures, GV, and OSI, helped expand deployment of its platform in clinical and research settings.
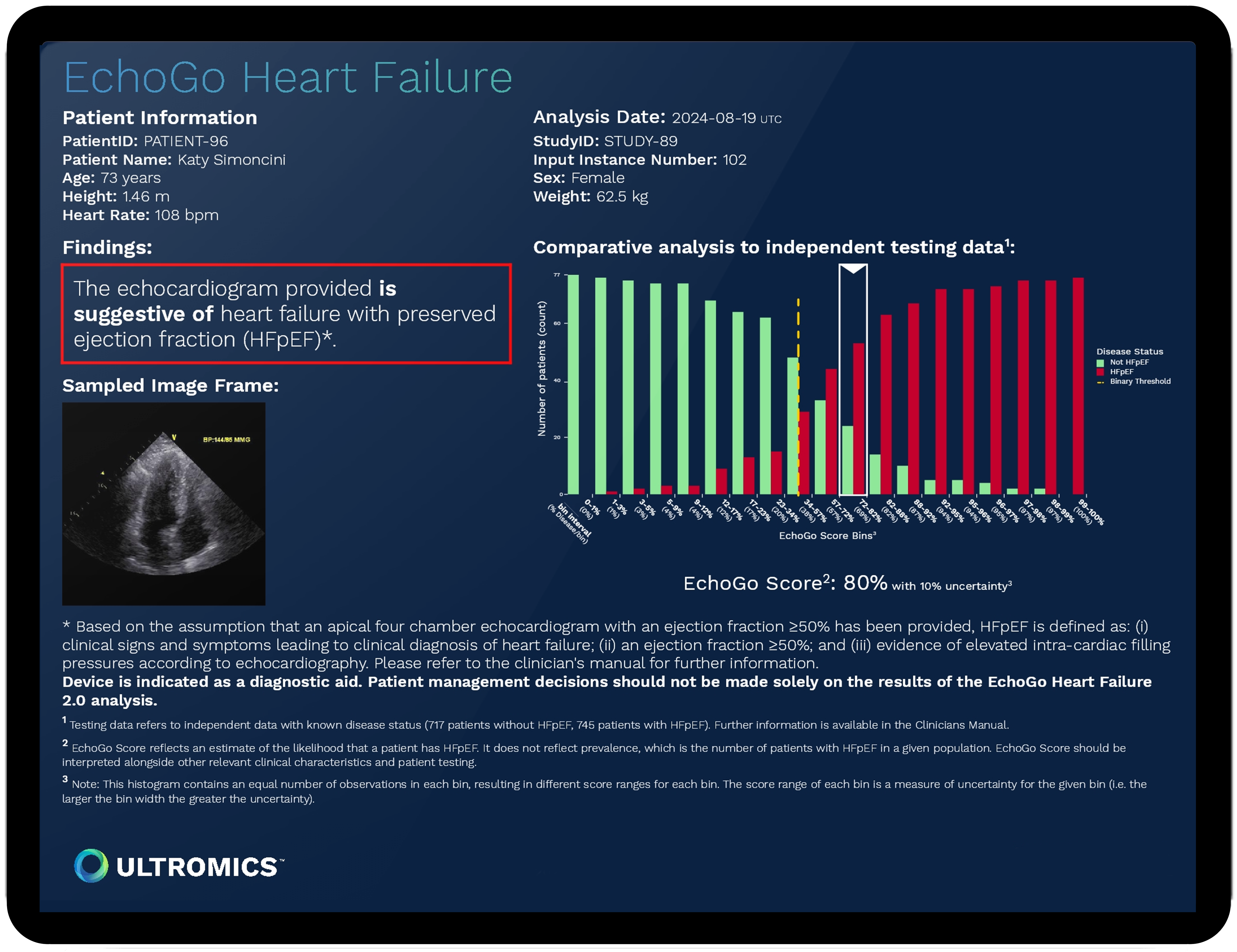
EchoGo Heart Failure; Source: Ultromics
Ultromics’ flagship platform, EchoGo, has reportedly analyzed over 430,000 echocardiograms to date. In clinical validation, EchoGo improved HFpEF detection by 73% over standard clinical risk scores. The company also received breakthrough designation and FDA clearance for EchoGo Amyloidosis, which achieved 85% sensitivity and 93% specificity in distinguishing cardiac amyloidosis from similar conditions in a study published last month in the European Heart Journal.
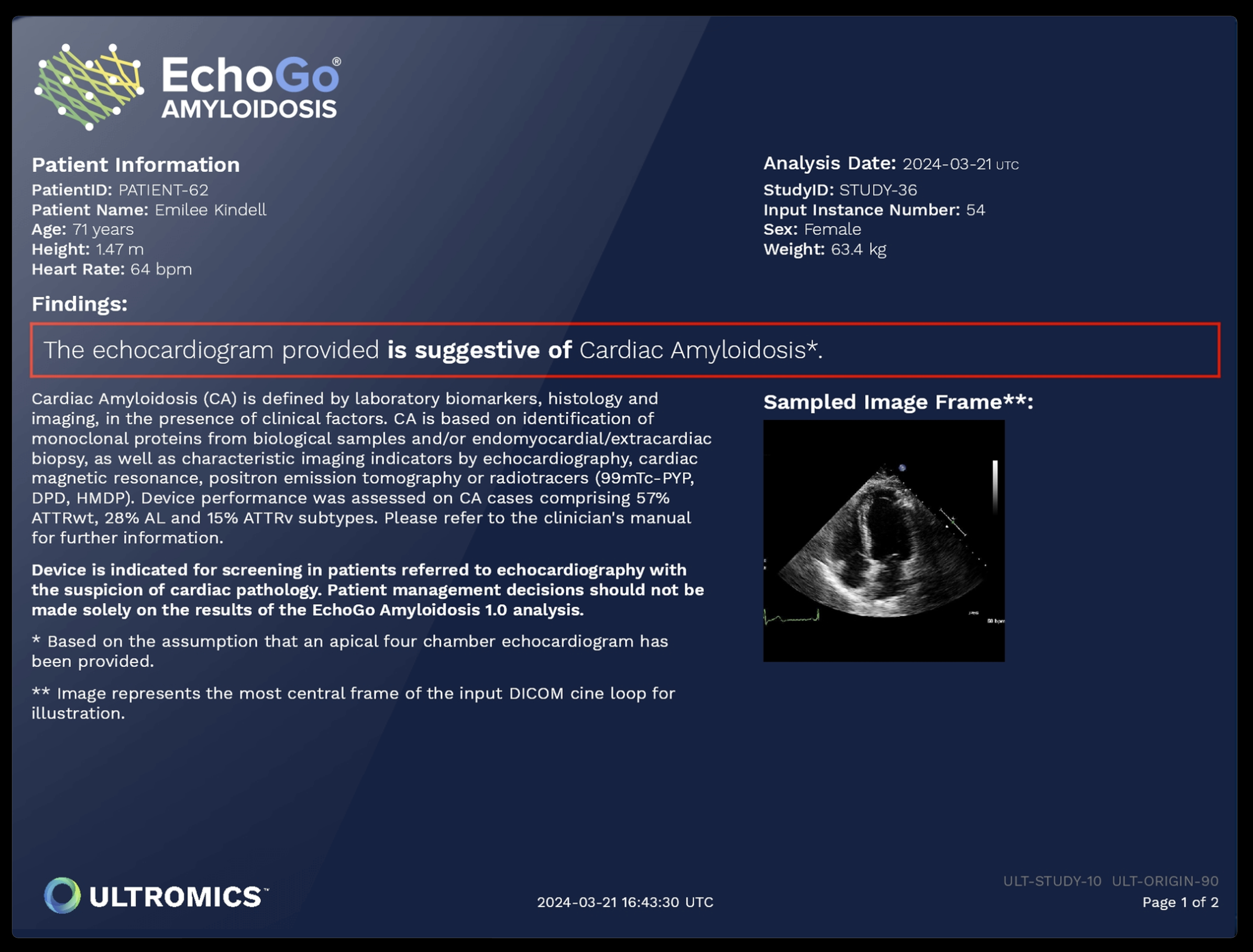
EchoGo Amyloidosis; Source: Ultromics
EchoGo Amyloidosis AI analyzes echocardiogram video loops using deep learning models trained to recognize myocardial motion abnormalities associated with amyloid deposition. According to a March 2025 Nature Communications paper, the model was developed on over 8,000 scans and validated on 2,600+ patients across multiple international centers.
The Series C round was co-led by L&G, Allegis Capital, and Lightrock, with participation from GV, Oxford Science Enterprises, Blue Venture Fund, UPMC Enterprises, UChicago Medicine’s UCM Ventures, and the University of Oxford. Johnson & Johnson’s Janssen Biotech and Pfizer previously supported development and validation of Ultromics’ amyloidosis tool.
The company plans to use the new funding to scale its commercial footprint amid rising demand for precision diagnostic tools in cardiology.
Topics: Startups & Deals