Neuralink Begins UK Clinical Trial of Brain Implant for People with Paralysis
Neuralink, Elon Musk’s brain computer interface (BCI) developing company, has launched its first clinical trial in the UK to evaluate the safety and functionality of its N1 brain-computer interface (BCI) implant. The feasibility study, called GB-PRIME, will enroll up to seven participants with severe neurological conditions—such as spinal cord injury or motor neurone disease—that impair the ability to use digital devices. The trial is being conducted at two sites: University College London Hospitals (UCLH) and Newcastle Upon Tyne Hospitals.
See also: Beyond Neuralink: The Diverse Landscape of Brain-Computer Interfaces
The N1 implant is a wireless, fully implantable BCI designed to allow users to control external devices, such as smartphones or computers, using brain activity alone. The device captures neural signals through over 1,000 electrodes embedded in ultra-thin threads, each finer than a human hair. These are precisely placed within key areas of the brain using Neuralink’s R1 surgical robot under the supervision of a neurosurgeon.
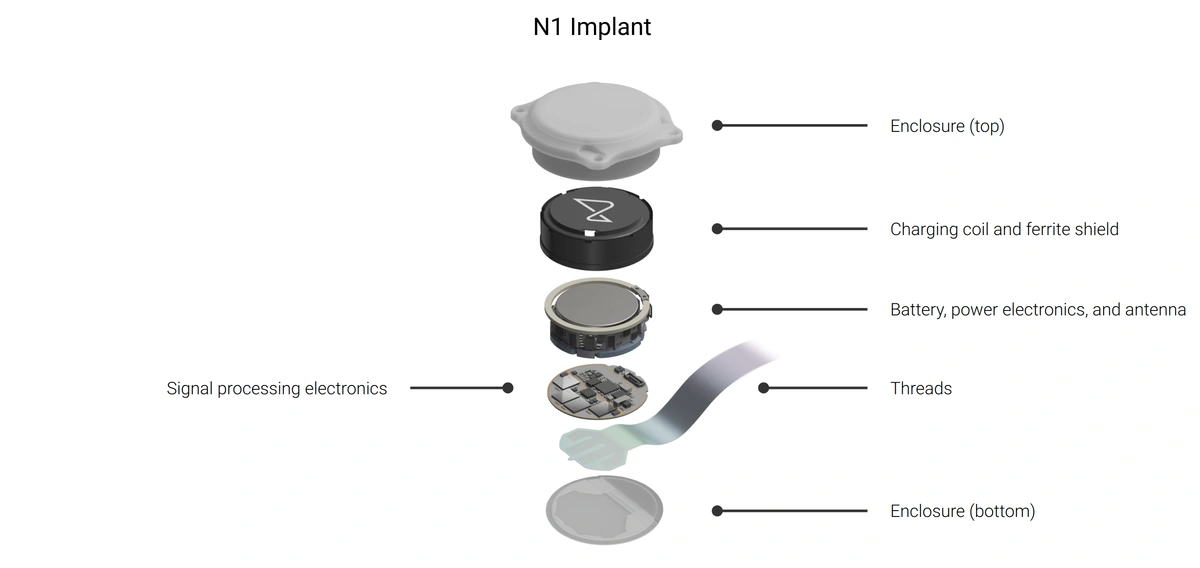
Image: Neuralink’s R1 surgical robot
Participants will undergo surgery at UCLH’s National Hospital for Neurology and Neurosurgery, with trial oversight provided by researchers at UCL and Newcastle University. The trial has been approved by UK regulatory bodies, including the Medicines and Healthcare products Regulatory Agency (MHRA), the Health Research Authority (HRA) and Health and Care Research Wales (HCRW), and a national ethics committee.
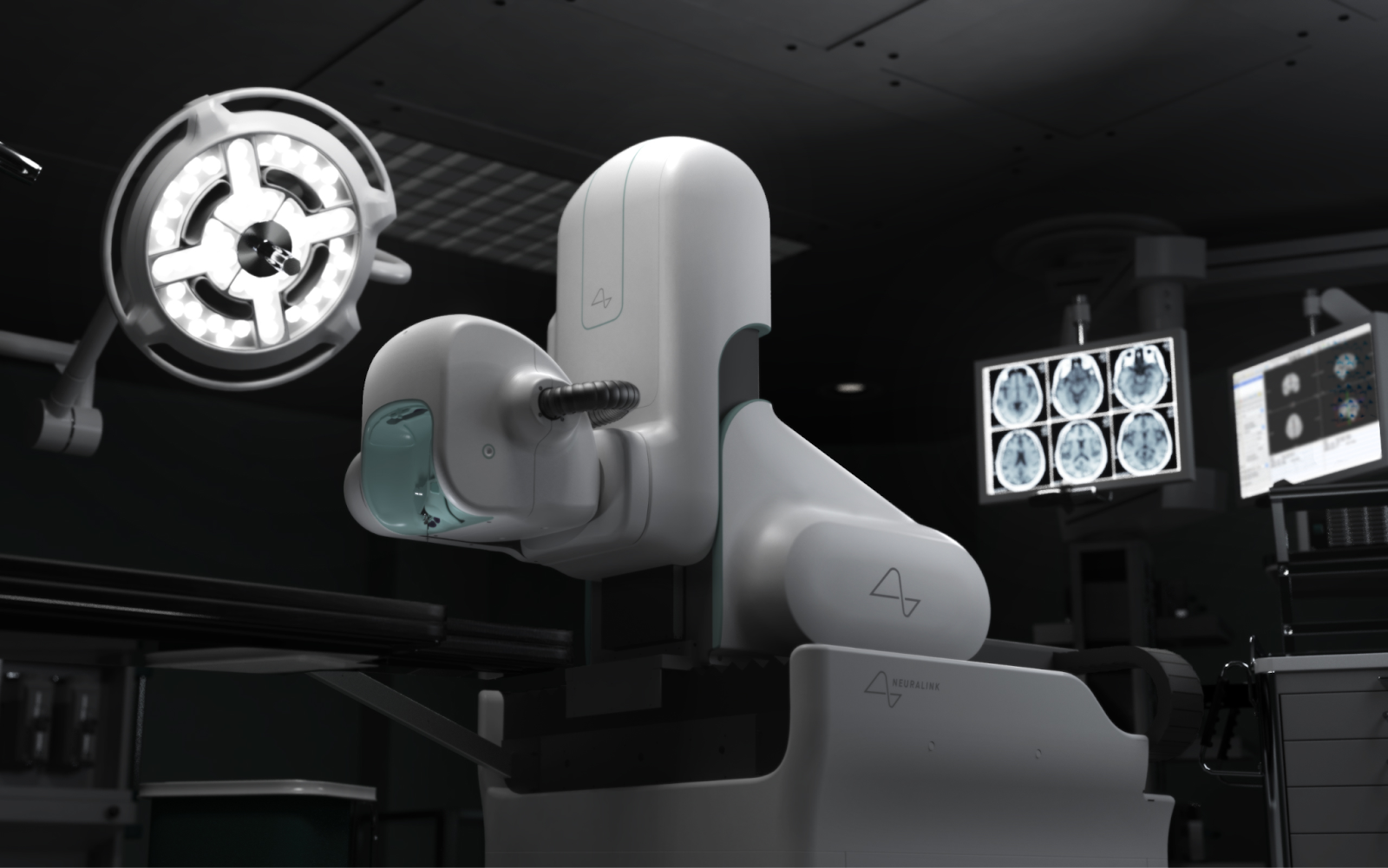
Image: Neuralink's N1 implant structure
GB-PRIME follows similar trials in the US, Canada, and the UAE, where at least nine participants have already received implants. In the UK, eligible participants include individuals over 22 years old living with paralysis caused by conditions such as spinal cord injury and amyotrophic lateral sclerosis (ALS).
At this time, the GB-PRIME study will not include the use of BCI for robotic arm control. Interested individuals can register at neuralink.com/trials.
Topics: Clinical Trials