AI-Designed Drug Reaches Clinical Validation: Insilico’s Rentosertib in Nature Medicine
Today, Nature Medicine published peer-reviewed Phase IIa clinical results for Rentosertib (ISM001-055), a TNIK inhibitor developed by Insilico Medicine using a generative-AI workflow. The paper details the GENESIS-IPF trial—a randomized, double-blind, placebo-controlled study of Rentosertib in idiopathic pulmonary fibrosis (IPF)—and the same data were presented at the American Thoracic Society 2025 meeting.
71 patients were enrolled at 22 sites in China and assigned to placebo or one of three dosing regimens: 30 mg once daily, 30 mg twice daily, or 60 mg once daily. After 12 weeks, the 60 mg group showed a mean forced vital capacity (FVC) increase of +98.4 mL, versus a mean decline of −20.3 mL in the placebo group. The dose-dependent FVC trend supports a pharmacological effect.
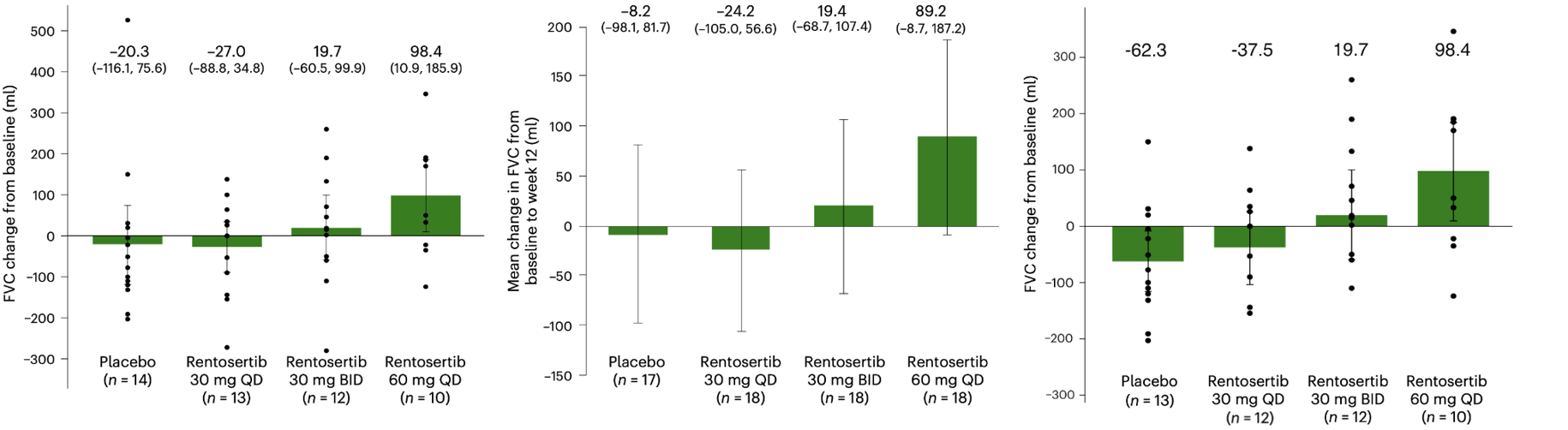
Absolute changes in FVC (±95% CI) after 12 weeks of Rentosertib treatment.
Left: Observed changes from baseline. Center: ANCOVA model with multiple imputation under MAR assumption. Right: a supplementary panel from Insilico with sensitivity analysis excluding two outliers with >600 mL discrepancy between screening and baseline FVC.
The trial met its primary safety endpoint. Adverse events occurred at similar rates across groups; most were mild or moderate, and serious events were rare and resolved after treatment discontinuation.
"These results not only suggest that Rentosertib has a manageable safety and tolerability profile but also warrant further investigation in larger-scale clinical trials of longer duration" said Alex Zhavoronkov, PhD, CEO of Insilico Medicine; the company has begun discussions with regulators to enable prospective evaluation in broader patient populations.
Exploratory serum-protein profiling revealed dose- and time-dependent biomarker changes consistent with Rentosertib’s proposed anti-fibrotic and anti-inflammatory mechanism: profibrotic markers such as COL1A1, MMP10, and FAP decreased, while IL-10 increased, correlating with FVC improvements. Lead investigator Dr. Zujun Xu emphasized that although the findings are encouraging, the limited sample size calls for validation in larger cohorts.
Rentosertib was conceived on Insilico’s end-to-end AI platform, which identified TNIK (Traf2- and NCK-interacting kinase) as a fibrosis driver and generated molecules to inhibit it. IPF affects roughly 5 million people worldwide and carries a median survival of three to four years; current antifibrotics slow progression but do not reverse it. These Phase IIa data support further evaluation of Rentosertib as a potential disease-modifying therapy.
Insilico Medicine’s Phase IIa results for ISM001-055 were first highlighted in a clinical news dispatch in September 2024, followed by a more detailed contextual analysis in November 2024 that examined the therapeutic relevance, study design, and the role of generative AI in the drug’s development.
Topics: Clinical Trials