Is There an "Innovator's Dilemma" in Biotech?
The term "Innovator's Dilemma" was coined by Clayton Christensen in 1997. The Innovator's Dilemma refers to a situation where successful, well-established companies face challenges in adopting and integrating new and innovative technologies or business models, despite their proven success in the existing market.
I often hear from biotech executives that they also face a dilemma.
Biotech executives often find themselves grappling with a similar predicament, particularly when dealing with innovative new molecular entities (NMEs).
Most biotech innovations revolve around innovative NMEs—active substances with entirely new mechanisms compared to established small molecules or antibodies. This includes revolutionary technologies such as molecular glues, PROTAC, CRISPR, or mRNA.
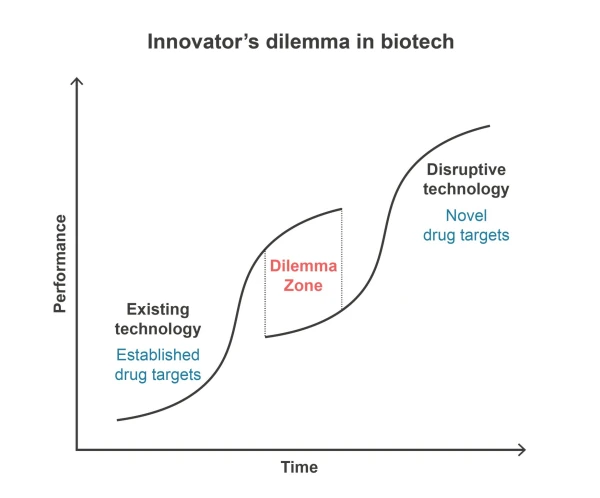
Developers of innovative NMEs face a critical decision: whether to focus on established drug targets or venture into uncharted territories. The dilemma lies in the perceived risk of testing NMEs in new biological contexts. Should proof-of-concept be demonstrated in a familiar, established field before branching into new ones?
I hold a clear opinion on this matter and am eager to engage in discussions here:
In my view, there is no dilemma.
NMEs should be developed for new drug targets, not against established ones.
Why?
Because, as highlighted by the Boston Consulting Group (BCG)'s analysis, "First-in-Class" typically outperforms "Best-in-Class" in the market: https://www.nature.com/articles/d41573-023-00048-2
"First-in-Class" underscores the novelty and uniqueness of a drug's mechanism of action, while "Best-in-Class" focuses on superior efficacy, safety, or overall performance within a specific category or therapeutic class.
It's crucial to recognize that drug development inherently carries risks. What I find noteworthy is that our efforts often center on minimizing this risk, inadvertently limiting the potential reward if the innovation proves successful.