Proscia Launches New AI Pathology Platform to Link Patients With Drug Trials
Proscia, headquartered in Philadelphia, has introduced Aperture, a pathology-based AI system that links diagnostic laboratories with biopharmaceutical companies to accelerate precision medicine. The platform is designed to identify eligible patients at the point of diagnosis and generate data to support clinical trial enrollment, regulatory submissions, and companion diagnostic development.
Aperture operates on Proscia’s Concentriq enterprise pathology platform. Concentriq serves as the digital foundation for pathology, unifying data from tissue images, molecular results, and clinical records. Its cloud-based design enables laboratories to digitize workflows, deploy multiple AI models simultaneously, and integrate third-party tools via APIs. In January 2025, the company reported 2.4 million patients diagnosed via its AI pathology platform, with expectations of surpassing 8 million in 2025.
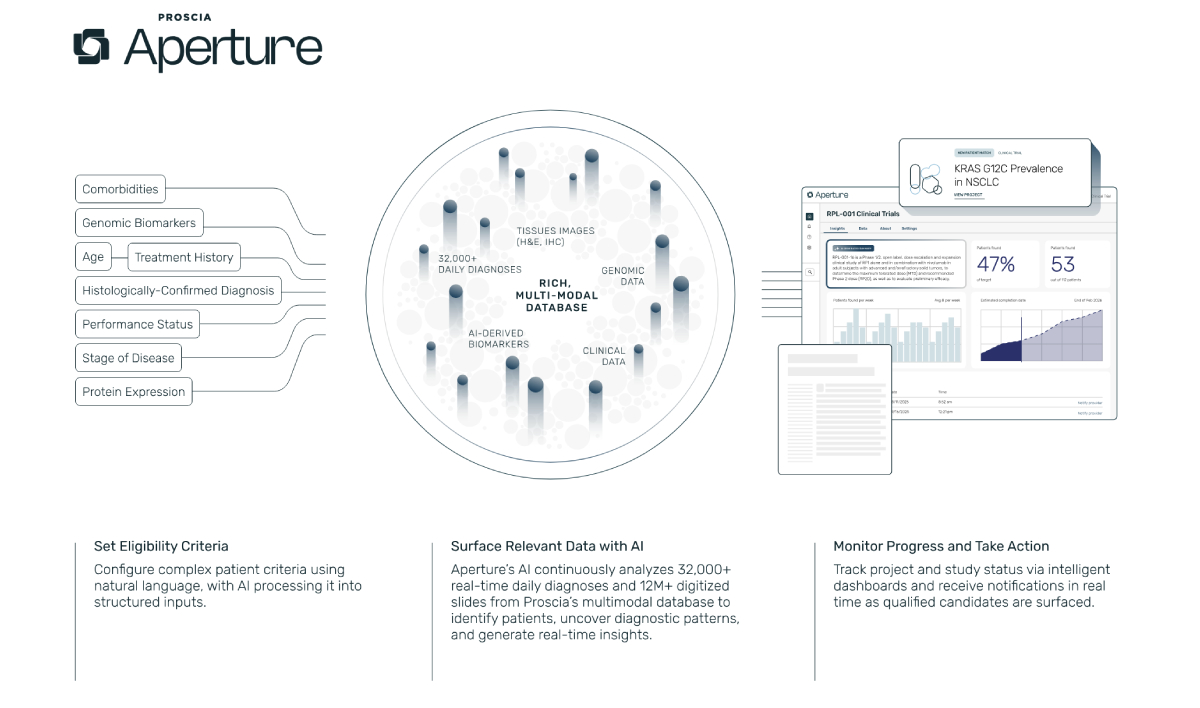
Image credit: Proscia Aperture
Earlier this year, Proscia raised $50 million in new funding—bringing total capital raised to $130 million—to expand Concentriq’s AI capabilities, enhance biomarker discovery, and broaden adoption across pharmaceutical and diagnostic partners. The platform is reportedly in use by 16 of the 20 largest pharmaceutical companies and processes over 22,000 patient diagnoses each day.
The latest update to Concentriq AP introduced multi-AI workflows, allowing pathologists to review outputs from several algorithms within a single interface for cancers such as breast, colon, gastric, lung, and prostate.
Aperture builds on this infrastructure by connecting to a diagnostic network projected to handle more than 8 million pathology cases each year. It uses these routine diagnoses to generate real-time insights, identifying trial-eligible patients shortly after diagnosis—typically earlier than genomics or EHR-based approaches. The system also leverages Proscia’s multimodal database of more than 12 million digitized slides linked to clinical and genomic data, which is used to refine biomarker strategies, identify novel drug targets, and generate regulatory-grade real-world evidence.
Biopharma companies can apply these datasets across the drug development cycle: modeling biomarker prevalence to guide early trial design, predicting enrollment feasibility, and producing evidence for label expansion and payer negotiations. For diagnostic laboratories, Aperture creates new revenue opportunities by integrating them directly into precision oncology pipelines and positioning them as strategic partners in clinical research.
By embedding AI at the point of diagnosis, Proscia aims to address the persistent challenge of low clinical trial participation, as according to the company, fewer than 10 percent of cancer patients enroll in studies. Aperture’s combination of scale, diversity, and AI-driven enrichment is designed to help sponsors identify rare or biomarker-defined populations, recruit more representative cohorts, and accelerate precision drug development.
Topics: AI & Digital