A Market Review of DNA-encoded Libraries Technology in Drug Discovery
(Last updated: October 05, 2022)
The interest of pharma organizations in DNA-encoded chemical libraries (DEL) technology has been growing over the years, with numerous pharma organizations now having their internal screening programs using DELs or outsourcing capabilities from specialized DEL providers.
This report provides a bird's view of the DEL market, including a summary of DEL technology benefits and limitations, key players, major research deals, and several examples of successful hit discovery programs using DELs.
Historical context
The concept of DNA-encoded libraries (DEL) appeared in the early 1990th when scientists from Scripps Research Institute published a paper on “Encoded combinatorial chemistry.” The main principle of DEL technology is to encode each compound in the library with a unique DNA barcode. Thereby library of billion compounds can be screened as a mix in a single test tube, and the active compounds are identified by amplification and sequencing of the DNA barcodes.
At the initial stage, companies pioneering DEL development faced a lack of suitable methods for DNA sequencing. Scientists from Praecis Pharmaceuticals then founded a company that managed to sequence DEL libraries. Praecis and Nuevolution were the first to have taken a chance on DEL in the 2000s. In 2005 and subsequent years, second-generation sequencing emerged, making DEL a much more attractive and economically feasible approach to drug discovery.
An overview of DEL technology
DEL technology comes from the merge of DNA encoding and combinatorial chemistry. The most popular method to build DEL is the “split and pool” approach. At the first step of the synthesis, chemical building blocks (BBs) are tagged with DNA barcodes. In the second step, they are mixed and then split into different portions. Next, a new set of building blocks is added to the chemical reaction, and then corresponding DNA barcodes are attached and linked with previous DNA molecules. Thus, DNA encodes each step of chemical synthesis for each compound.
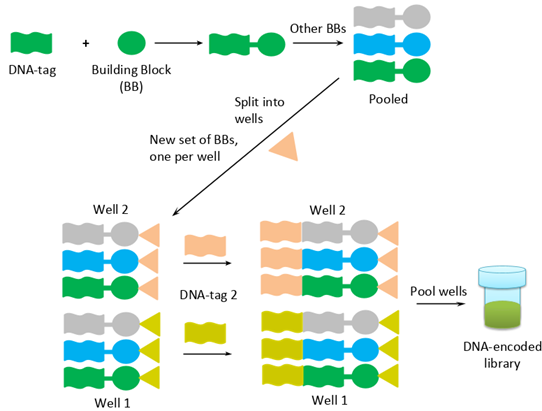
* Reproduced from C&EN
Size and costs of DELs
The “Split and pool” method enables to build of libraries containing up to 10^10 compounds. For example, HitGen’s libraries contain 400 billion molecules, X-chem provides 200 million compounds for screening, and Nuevolution has assembled a collection of 40 trillion compounds. To compare, the size of a common HTS library is restricted to several million compounds due to the prohibitive cost of synthesizing and managing more extensive collections. Overall, the cost of creating and screening an HTS library of 1 million compounds would cost millions of dollars (approximately $1,100 per compound), while screening a DEL library of 800 thousand compounds would cost on the order of $150.000.
Hit discovery using DELs
Screening of the DELs is conducted in single test tubes, where the target protein is incubated with a library. The target proteins can be anchored on a solid support, commonly magnetic beads. After thorough washing, only high-affinity ligands remain associated with a target. After DNA sequencing and decoding of the structure of active compounds, the last should be resynthesized to repeat the affinity binding assay. A lot of different selection strategies can be applied, even phenotypic screening is compatible with DELs.
DEL is suitable for the synthesis and screening of small molecules, macrocyclic compounds, and peptides. Scientists are harnessing the chemical properties of DNA, especially the principle of complementarity, to develop new strategies for library design.
DNA templated strategy
One of the more sophisticated methods to build libraries is to use DNA code to determine the sequence of chemical reactions. Templates are prepared before synthesis and contain regions complementary to barcodes of BBs. When barcodes couple to the template, the relative sterical arrangement of building blocks facilitates a chemical reaction between them. This method is advantageous when assembling libraries of macrocycles and peptides.
The alteration of the approach is used in Vipergen’s YoctoReactor.
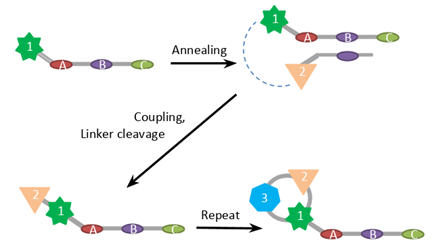
* Reproduced from FEBSPRESS
Dual-pharmacophore libraries
The advantage of dual-pharmacophore libraries is that two chemical moieties have the flexibility to reach adjacent non-overlapping binding sites of a target. Dual-pharmacophore libraries are assembled from two sub-libraries; when mixing, they form heteroduplexes representing two compounds. ESAC (Encoded Self Assembling Chemical) is the most popular method.
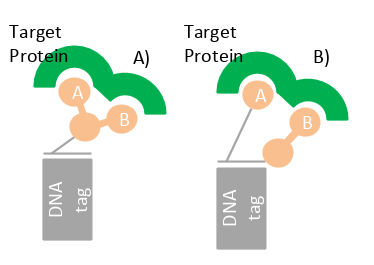
* Reproduced from FEBSPRESS
DEL-compatible chemistry
The chemistry applicable to DEL technology is restricted to reactions compatible with DNA. DNA is a water-soluble molecule, thereby traditional organic reactions should be translated to corresponding conditions. In the beginning, only a few reactions were available in chemists' toolbox, but their number is extended, harnessing various synthesis approaches. One of the recent ideas was to employ photoredox catalysis for DNA‐Encoded Chemistry.
Pros and Cons of DNA-encoded Libraries Technology
| Benefits | Limitations/challenges |
| Large size (up to 10^10 molecules available for screening) | Limitations in types of BBs and reactions that can be used (has to be compatible with DNA chemistry) |
| Might be applied to “undruggable” targets | Oligonucleotide might affect binding affinity |
| Low costs and time of screening compared to HTS | Targeting DNA/RNA-binding proteins is challenging. |
| Less target amount is needed when compared to HTS, relatively simple assay methods. | Need to resynthesize active molecules to determine their affinity |
| Library refinement capabilities, flexibility. | Challenges with signal-to-noise ratio when selecting active compounds |
Market and trends
HitGen and X-Chem are two major providers of DEL capabilities. Both companies leverage the “split and pool” approach to construct libraries. They have announced the more significant part of DEL collaborations with pharmaceutical companies. These two companies offer their partners to screen ready-to-use DELs, or build libraries for their needs. HitGens libraries grew up to 1 trillion novel drug-like molecules compared to 400 bln in 2019. They offer a wide range of end-to-end services in drug development, starting from target validation and hit identification and finishing with clinical outcomes. In September 2022, HitGen entered a research collaboration with a US-based company Unison Medicines to develop anti-infectives. The bacterial target they are working on will be disclosed later.
Several pharmaceutical giants such as GSK, Novartis, and Amgen, are developing DEL primarily for their own targets of interest. GlaxoSmithKline was the first company to adopt the technology. In 2007 GSK acquired Praecis Pharmaceuticals (a pioneering company) after the successful collaboration in 2006, which eventually led to 2 compounds getting their way to clinical trials. The clinical studies were suspended at the Phase II stage because of the adverse effects. In 2021 GSK also published their achievement in developing the highly selective inhibitor GSK040 for the bromodomain and extra terminal (BET), which has an important application in cancer immunotherapy.
A similar story occurred with Amgen and Nuevolution, which started collaboration in 2016. Three years of collaboration have led to an acceleration of the progress of two cancer programs. In 2019 Amgen acquired Nuevolution for $167 million to advance its drug discovery efforts. They used the advancements in DEL technology to work on protein degradation PROTACs and enrich their cancer pipeline with the new drug candidates in general.
The number of partnerships announced for DEL technology keeps on growing, with increased growth dynamics starting from 2014 (BPT Analytics). This year was particularly fruitful for the DEL market due to the activity of Vipergen, X-chem, and Nuevolution. HitGet, founded in 2012, went on a deal spree starting in 2017 with more than a dozen deals with various partners. One of the latest deals includes the research collaboration between HitGen and Howard Hughes Medical Institute with Duke University, announced in March 2022. By using HitGen’s DELs platform, they aim to perform selections against GPCR targets. Shortly after this deal, Vipergen announced a collaboration with LEO Pharma in June 2022 to use DELs to discover novel treatments for dermatology indications.
Behind acknowledging the activity of large brands in the DEL space, several startups have been founded over the last few years, including Haystack Sciences (received $4.2M series A financing in October 2019) and DyNAbind GmbH (raised seed round in 2019). Haystack Sciences was afterward acquired by the AI-driven drug development company Insitro in late 2020. A US-based Plexium now has total funding of $165M, with the latest $102M Series B round closed in February 2022.
The technology seems to be developed to such a level that several companies are providing DEL kits for library screening. In September 2019, WuXi AppTec announced the launch of DELlight platform, which would supply customers with DEL kits containing 10,8 billion compounds and 58 libraries together with operational protocols for performing the procedure. WuXi AppTec also offers a smaller-size DELopen Kit for academic users. With the use of blockchain technology, DELopen offers maximum IP protection for users who utilize the online platform. DyNAbind's kits are available for around 12,5 thousand EUR at Sigma-Aldrich.
Interestingly, in 2022 an agriculture-oriented company Enko Chem acquired proprietary DELs from X-Chem, planning to utilize this technology for agricultural applications. The libraries can help to accelerate the discovery and development of sustainable crop protection solutions.
Smaller companies and startups
Small companies and startups harnessing DELT are worth considering in more detail. They exploit DEL approach for several notable use cases, including ubiquitin-proteasome system (UPS), allosteric regulatory sites, PPI, and multi-drug resistant bacterial infections. Below is a brief reference to several advanced combinations of DELs with other technologies.
DEL in phenotypic screening -- protein degradation
Plexium’s DELPhe platform enables cell-based phenotypic screening of DNA-encoded libraries. Their technology miniaturizes screening to the scale of picoliter volumes. Plexium is focused on developing modulators of E3 ligases, working on a targeted protein degraders technology - PROTACs. The company also has a well-established AI platform used for genomic analysis and drug design. In April 2022, Abbvie collaborated with Plexium to develop targeted protein degradation therapies for neurological conditions.
DEL for studying allosteric sites
HotSpot Therapeutics is focused on developing drugs that interact with allosteric sites - “regulatory hotspots.” In 2014, they acquired Macroceutics, Inc, a provider of DEL screening technologies to advance HotSpot's SpotFinder™ platform, which can be valuable in predicting and evaluating different allosteric regulatory sites. HotSpot Therapeutics’ Smart Allostery™ integrates machine learning, structure-function, and tailored chemistry to uncover potential drug pockets on proteins. Their allosteric drugs are mostly validated in the preclinical models, like CBL-B inhibitors with differentiated immune-enhancing activity.
In summary, most of the DEL platforms can roughly be classified into four categories:
-
commercial DEL platforms
-
in-house DEL platforms for own programs
-
open-access DEL platforms
-
standardized DEL kits
Some of the notable DELT successes to date
Besides the number of deals and emerging companies, the pipeline derived from DELs is no less encouraging. The first example is Nuevolution’s BET-BD1 program. BD1 is the first bromodomain of the BET family, which is responsible for epigenetic regulation of the immune system. A candidate molecule NUE20798 started a clinical trial in 2019 against atopic dermatitis, but the studies were not continued further. Previously, inhibitors of this class have not been approved. Another successful example came from a partnership with Almiral in 2016: RORγt inverse agonist entered the clinic to treat psoriasis (PsO) and psoriatic arthritis in 2019.
A drug candidate X-165 has been accepted by FDA for Phase 1 clinical trial. This is a highly potent inhibitor of autotaxin owned by X-Rx. This company spun out from X-chem and used its DEXtm (based on DEL technology) platform for drug discovery. Since 2012, X-chem has licensed 50 drug discovery programs.
HitGen got approval from FDA for the clinical trial of a drug candidate against multiple myeloma - incapsulated selective HDAC inhibitor. Three other drug candidates for solid tumor treatments were approved for clinical trials, but the company also developed other molecules against different targets in their pipeline.
GSK owns a specific inhibitor of the RIP1 kinase GSK2982772 and completed a Phase II clinical trial for psoriasis. Another inhibitor of the soluble epoxide hydrolase (sEH) GSK2256294 is currently wrapping up Phase II testing, which is expected to be finished in October 2022. GSK scientists are known to be the first who describe the cell-based DNA-encoded library screening in 2015. They used the cell line with an overexpressed receptor neurokinin-3 (NK3), and the cells were incubated with roughly 15 billion DEL compounds, after which binding molecules were eluted by the cell denaturation. Such an experiment setup allowed the identification of multiple NK3 antagonists from several DEL libraries, and some of the hits were further optimized.
Vipergen pushed this technology even further and, at the beginning of 2021, presented a Cellular Binder Trap Enrichment (cBTE) to screen DELs inside the living cells. To do so, they inject DELs inside the living cell - the Xenopus Laevis oocytes, which are 100 000 times bigger than somatic cells and hence suitable for microneedle manipulations. When the protein of interest is expressed in the oocytes, it is fused to a “prey” protein domain. A “bait” is a DNA conjugate that binds the prey protein when it is injected with the DEL library into oocytes. As a result, the formed bait-prey complex will be detected with PCR amplification.
Topic: Next-Gen Tools