Crown Bioscience Shows 3D Bone Marrow Model to Improve Blood Cancer Drug Testing and Reduce Animal Use
At the 2025 Annual Meeting of the American Association for Cancer Research (AACR)—a major international conference focused on cancer science and therapeutic development—Crown Bioscience, a preclinical contract research organization specializing in oncology and immuno-oncology, announced the launch of its 3D Bone Marrow Niche (BMN) platform.
See also: FDA Plan to Replace Animal Testing with AI Models and Human-Based Methods in Drug Evaluation Shift
Traditional 2D cultures and scaffold-free suspension assays have limited ability to replicate the complex bone marrow microenvironment, which plays a central role in disease progression, immune evasion, and therapeutic resistance in blood cancers. Crown’s BMN platform is designed to more closely reflect this native architecture by incorporating key bone marrow components (stromal cells, endothelial cells, and mesenchymal-endothelial networks) within biofunctional hydrogel scaffolds.
According to the company, this structure supports hematopoietic stem and progenitor cell (HSPC) expansion and allows for interactions with autologous or heterologous immune cells. The resulting environment aims to sustain primary cell viability, maintain lineage-specific behavior, and enable better modeling of cell–cell and cell–matrix interactions.
The platform is described as optimized for acute myeloid leukemia (AML) and multiple myeloma (MM), and adaptable for broader use in hematological toxicity screening and immune interaction studies.
Hydrogel-Integrated 3D Niche Design
The 3D BMN platform uses a composite system of mesenchymal and endothelial cells embedded in functionalized hydrogels. These hydrogels serve as a structural and biochemical scaffold intended to mimic the extracellular matrix and support physiologically relevant growth conditions.
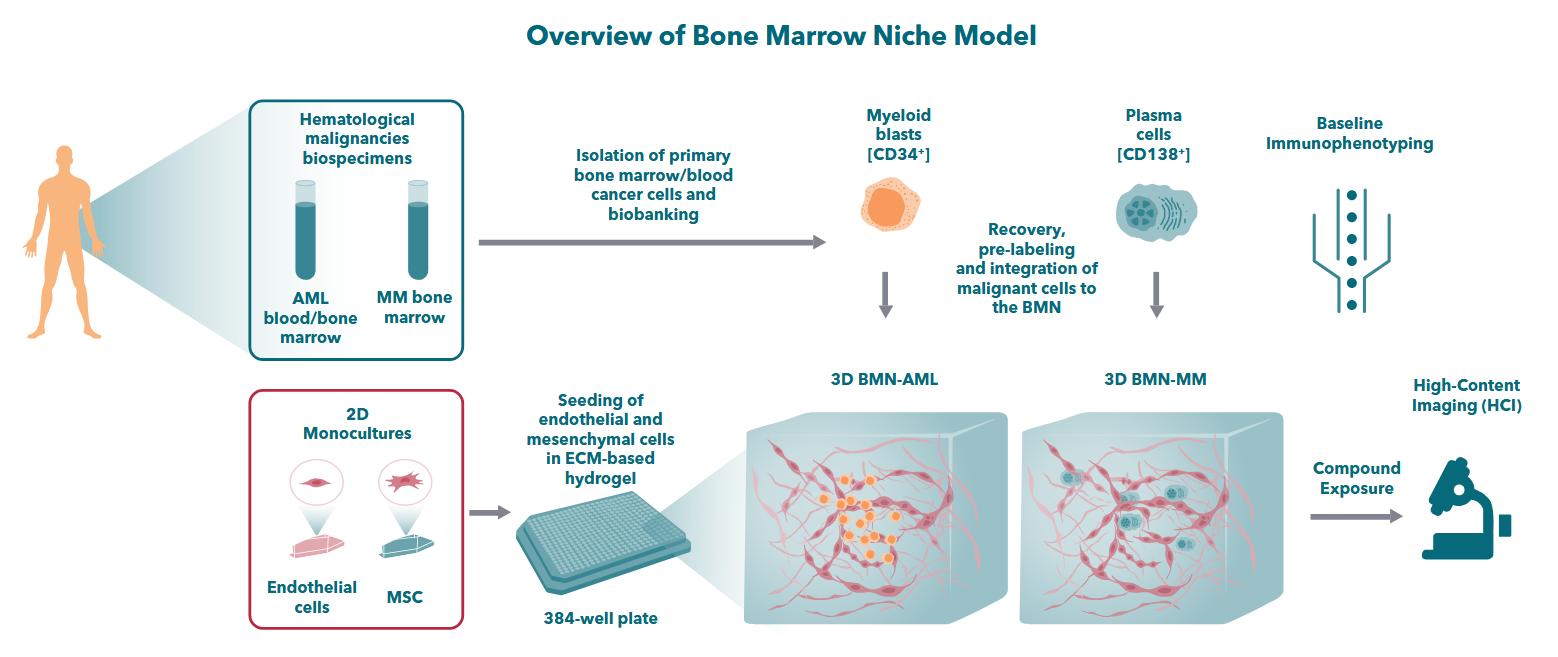
From an overview of Crown Bioscience’s 3D Bone Marrow Niche models, designed to replicate bone marrow-tumor interactions in vitro for studying hematological malignancies, drug resistance, and therapeutic response. Source: Crown Bioscience brochure
Key technical features include:
- Compatibility with patient-derived tumor samples and cell lines, to allow for disease-specific or individualized testing
- Inclusion of immune components, to support immuno-oncology applications and to enable co-culture with immune cells
- Pre-vascularization network simulation, which may enhance stem cell maintenance and differentiation
- Support for high-content imaging, to enable detailed analysis of drug response, cell viability, and immune cell interaction
- Application in hematopoietic toxicity screening, drug resistance modeling, and stem cell-based therapeutic development
Designed for use in 384-well formats, the platform can integrate into broader workflows (e.g. downstream in vivo studies) by offering more predictive ex vivo results. Crown suggests that this may reduce reliance on animal models and help derisk early-stage therapeutic candidates.
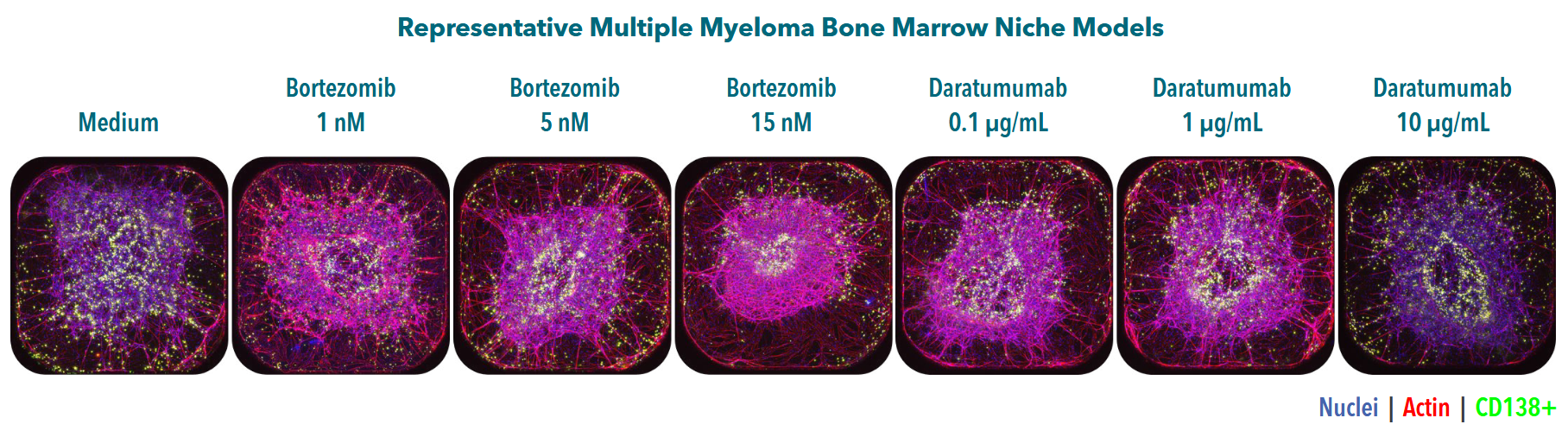
The platform supports a range of readouts including flow cytometry, high-content imaging, immunofluorescence, and optional single-cell RNA sequencing. Researchers can assess compound effects on both tumor viability and microenvironmental response (such as cytokine release or stromal remodeling) within the same system.
Drug resistance mechanisms, including cell adhesion-mediated drug resistance (CAM-DR), can be investigated by observing how malignant cells respond to standard therapies when embedded within ECM-rich conditions. These models are particularly relevant for studying leukemic and myeloma progression in bone marrow–like contexts.
The BMN platform is supported by Crown’s access to well-characterized patient-derived biobanks, which include clinical metadata such as treatment history and genomic profiles. These repositories provide primary tumor, bone marrow, and blood specimens for assay setup, enabling studies to reflect patient heterogeneity and real-world treatment contexts.
Bridging In Vitro and In Vivo
Crown Bioscience is positioning the BMN model as an extension of its broader 3D modeling and hematological research capabilities. The company already offers PDX models, tumor organoids, and immune-competent in vivo systems. The BMN platform is intended to bridge the translational gap between in vitro screening and in vivo validation by offering a more biomimetic early-stage testing system.
"Until now, bone marrow research has lagged behind solid tumor research due to lack of relevant in vitro models", said Ludovic Bourré, Vice President of Research and Innovation at Crown Bioscience. "With these BMN technological advances, we are now able to help researchers understand how cancer survives therapies once it reaches the bone marrow."