Axiom Bio Launches with $15M to Replace Animal Testing with AI for Drug Toxicity Prediction
San Francisco-based startup founded by Brandon White and Alex Beatson has announced $15 million in seed funding from Amplify Partners, Dimension Capital, and Zetta Ventures, with participation from angels including Jeff Dean, Laksh Aithani, Barry McCardel, Alec Nielsen, Ari Morcos, Stef van Grieken, and Elliot Hershberg.
Axiom Bio, launched in late 2023, is working to minimize clinical trial failures linked to drug toxicity—particularly liver injury—by replacing traditional animal experiments with AI-driven predictions.

Axiom Bio co-founders, Brandon White and Alex Beatson
Co-founder Brandon White argues that animal testing is becoming obsolete, not just for ethical reasons, but because it's slow, expensive, and poorly predictive. White points out that although animal studies remain standard, more than 90% of drugs that pass them ultimately fail in human trials.
He sees AI, trained on large, multimodal datasets, as a faster, more human-relevant alternative. But adoption will depend on rigorous validation: blinded comparisons, transparent performance benchmarks, and integration into real-world workflows.
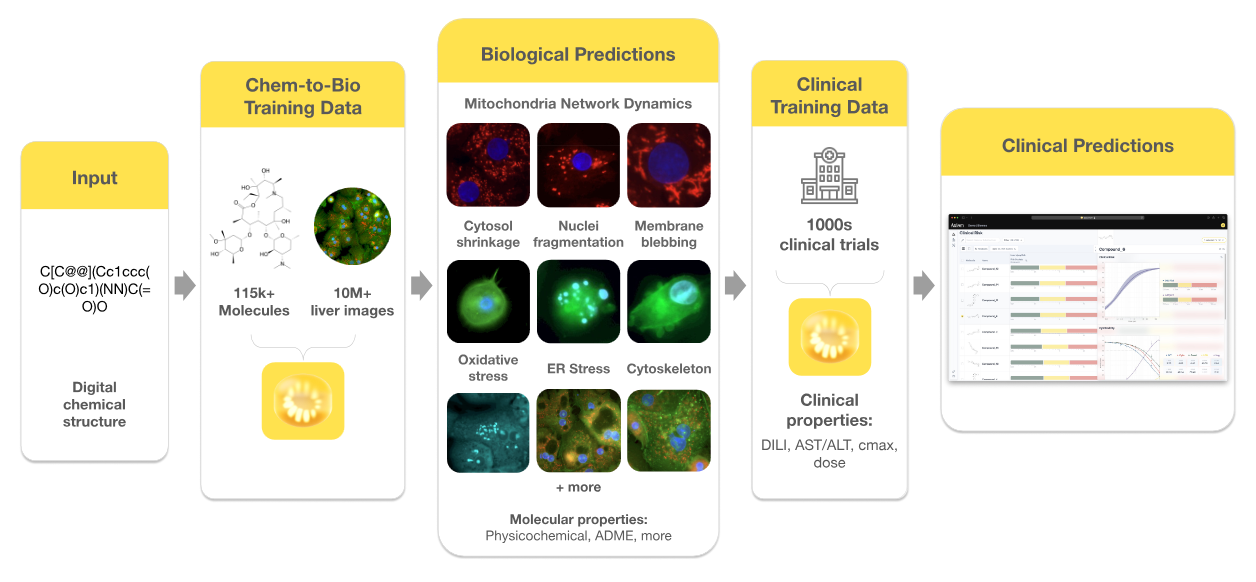
In its first year, Axiom built what it describes as the world’s largest human toxicity dataset by running proprietary assays on primary human hepatocytes and a multi-cell hepatic system, paired with high-content imaging and multiplexed biochemical readouts. According to co-founder Brandon White, this biologically rich approach distinguishes the dataset from public resources such as Tox21, which rely mainly on single-readout assays and non-primary cell lines.
Structure of Axiom Bio’s AI model for predicting drug-induced liver injury, from Society of Toxicology 2025 poster (PDF).
Dataset and Experimental Platform
Axiom’s dataset includes over 115,000 unique small molecules, 1,200 unique targets, and 50,000 unique scaffolds, as well as specialized compound classes such as macrocycles, PROTACs, and molecular glues. It also integrates clinical data on over 5,800 small molecules, including approximately 700 compounds from the FDA’s DILIrank database, providing a direct link between experimental results and known clinical outcomes.
Experimentally, Axiom operates two proprietary liver models:
- 2D primary human hepatocyte system with pooled donors, generating multiplexed high-content biochemical and imaging readouts after 48-hour compound exposures across dose ranges.
- 2D+ multi-cell hepatic system that incorporates hepatocytes with stromal and endothelial support cells, allowing for multi-dosing regimens over two weeks. This model enables evaluation of complex liver-specific phenotypes such as cholestasis and steatosis.
These efforts have resulted in the labeling of over 394 million individual cells and 9 billion mitochondria, supported by more than 7,000 hours of human curation work. In parallel, Axiom curated a large-scale clinical toxicity dataset with more than 5,200 documented DILI likelihood scores, over 38,000 liver enzyme elevation datapoints, and detailed human pharmacokinetic and pharmacodynamic profiles.
Liver Model Performance
Drug-induced liver injury (DILI) is responsible for roughly 20–25% of clinical trial failures, according to a 2015 study in Toxicological Sciences. Axiom reports that its AI model can identify toxic compounds with around 70–75% sensitivity, while maintaining about 90% accuracy in identifying safe ones. Its reported performance matches or exceeds that of lab-based assays used by Pfizer, AstraZeneca, and others, including both 2D cell cultures and 3D liver spheroid models. It also does so at a much lower cost: $100–$450 per compound, compared to $3,000–$15,000 for physical experiments.
Pilot Studies and Future Plans
Axiom’s liver toxicity model was introduced publicly at the Society of Toxicology (SOT) conference in March 2025 (Ewald et al 2025). According to White, the model has already demonstrated predictive performance in recent clinical trials, including toxicity risks identified for Pfizer’s Lotiglipron and Danuglipron programs and a safe profile predicted for Orforglipron.
Pilot studies for Axiom’s technology are currently underway or being finalized with a group of partners that includes six of the top twenty pharmaceutical companies, a major agrochemical company, multiple hedge funds, and several biotechnology firms. The platform can be deployed either securely in the cloud or managed on-premises
See also: FDA Plan to Replace Animal Testing with AI Models and Human-Based Methods
Axiom’s approach aligns with broader industry moves to phase out animal testing, following recent announcements by the FDA aiming to make animal studies "the exception rather than the norm".
Looking ahead, the company plans to expand its AI models to predict toxicity across additional organ systems, with models for brain, heart, and immunogenicity coming soon, alongside broader plans for kidney and other critical tissues. Axiom’s longer-term goal is to build a comprehensive whole-body human toxicity model.
Topic: Biotech Ventures