Phesi Reports GLP-1 Trials Now Span 100+ Diseases
Phesi has published an analysis showing that GLP-1 drugs are now being tested across more than 100 diseases, extending well beyond their established use in diabetes and obesity. The findings are based on 583 recruiting or soon-to-start trials tracked through the company’s Trial Accelerator platform and a newly published Digital Patient Profile (DPP) of GLP-1 patients.
Phesi’s DPP draws on records from ~1.9 million patients across 69,652 hospitals and clinics in 81 countries over 20 years. The analysis highlights growing clinical interest in GLP-1s as modulators of broader disease pathways, with trials spanning cardiovascular disease, polycystic ovary syndrome, osteoarthritis, and several cancers. In many cases, GLP-1s are being evaluated for their role in managing overlapping conditions such as hypercholesterolemia and hyperlipidemia, suggesting their potential utility in treating systemic clusters of disease rather than single indications.
Among the findings, it confirms a correlation between fasting plasma glucose and HbA1c, but finds no clear link between BMI and HbA1c, supporting evidence that body mass index alone is not a reliable measure of health.
Phesi’s review also found that obesity trials are taking longer to complete. Average cycle times have increased from 10–20 months two decades ago to 25–45 months today, reflecting both the complexity of study designs and growing competition for trial participants.
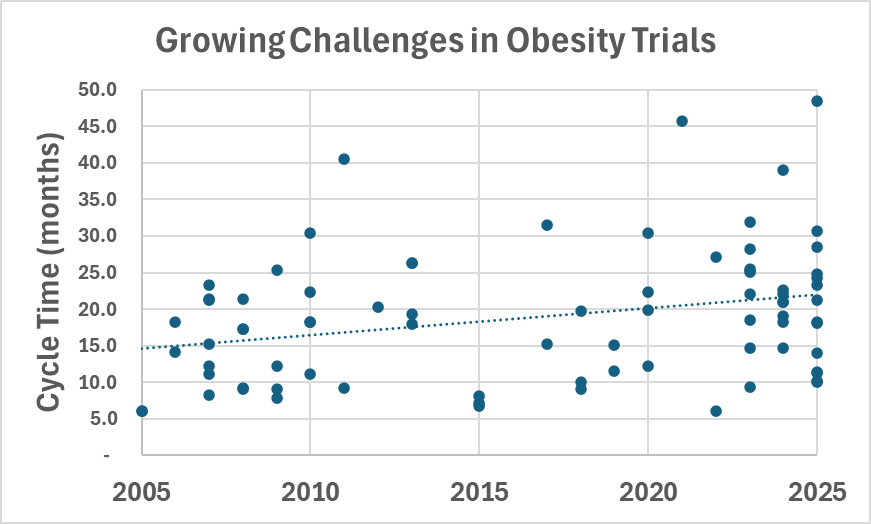
Image credit: Trend in clinical trial cycle times for obesity-related studies between 2005 and 2025, Phesi
Phesi’s Trial Accelerator, a software-as-a-service platform that supported the analysis behind the report, applies AI-driven analytics to a clinical trials database containing real-world data. The system provides predictive modeling for patient population identification, trial performance, country selection, and investigator site ranking. Its modules include patient profiling through Digital Patient Profiles, enrollment and cycle time simulations, and performance benchmarking across geographies and investigator sites, with the aim of reducing protocol amendments, shortening trial timelines, and improving data quality.
Earlier in 2025, Phesi published another large-scale study using its Trial Accelerator platform, analyzing 167 million patient records to map real-world data availability in oncology, pointing to uneven real-world data distribution and inefficiencies in trial execution.
Topics: Novel Therapeutics