Insilico Deploys its Chemistry Foundation Model on Microsoft Discovery Platform
Insilico Medicine reported the deployment of its Nach01 multimodal foundation model on Microsoft Discovery, Microsoft’s Azure-based platform for AI-driven research and development. The demonstration illustrates how third-party foundation models can be orchestrated within a single enterprise environment to support end-to-end computational drug discovery workflows, addressing fragmentation, reproducibility, and scaling challenges.
The integration embeds Nach01 within Microsoft Discovery’s agentic workflow framework, allowing models, data, and downstream tools to be composed into repeatable, multi-step pipelines. Microsoft Discovery provides workflow orchestration, identity and access management, and elastic compute via Azure ML, while Nach01 contributes generative and predictive chemistry capabilities, enabling molecule generation, property prediction, and downstream analysis within a unified system.
Nach01 is Insilico’s multimodal chemistry foundation model within its Pharma.AI platform, combining language-model-style architectures with structural and spatial representations. According to the company, it integrates textual and 3D spatial learning approaches from its earlier Nach0 model and is designed to support tasks including retrosynthesis, molecular property prediction, ADMET estimation, and candidate prioritization from hit generation through lead optimization. Insilico plans to publish Nach01 to the Microsoft Marketplace.
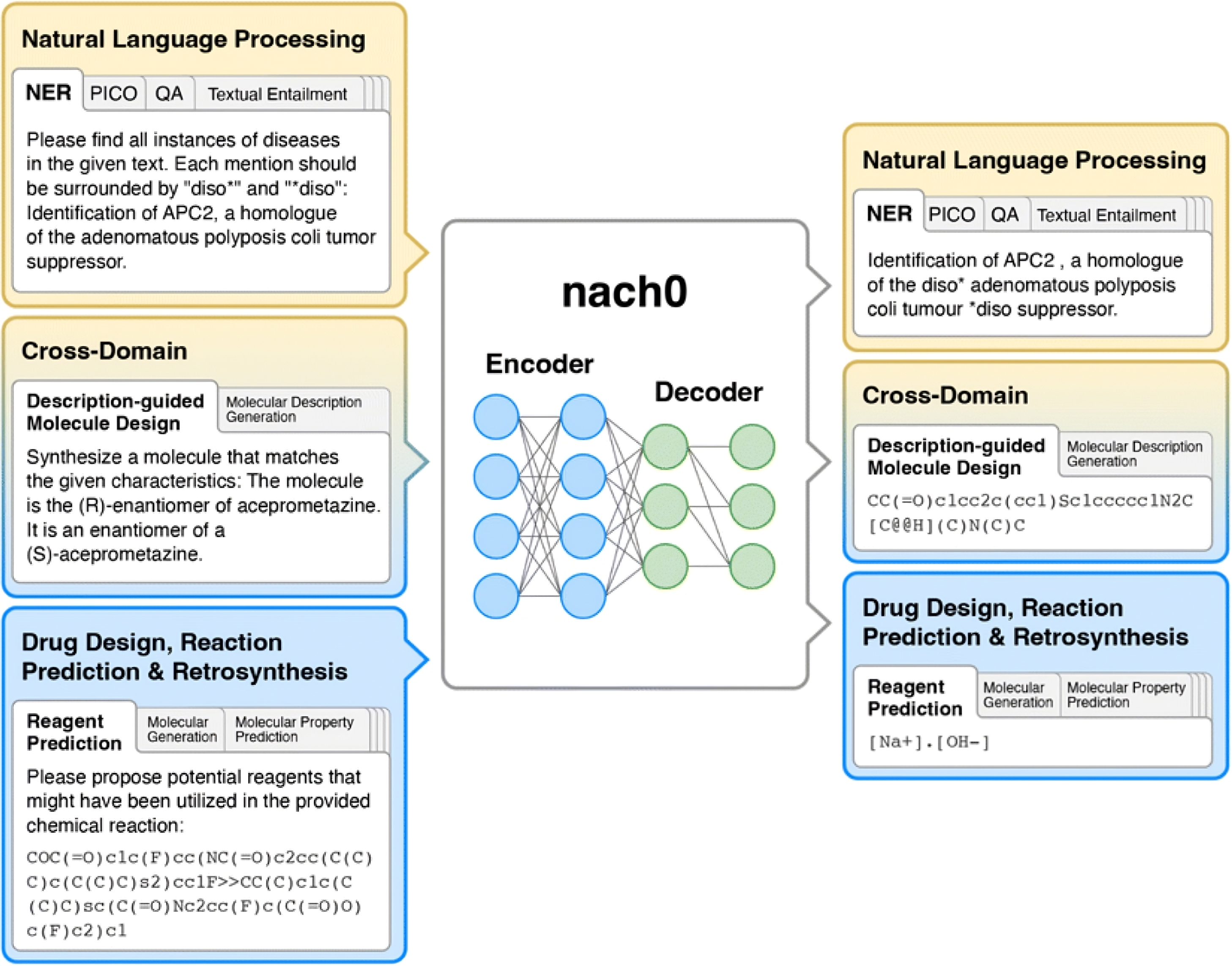
nach0 text-to-text framework, from Chemical Science (Issue 22, 2024). The model maps text inputs to task-specific target text using a unified architecture, loss function, and training setup across mono-domain (NLP, chemistry) and cross-domain (NLP–chemistry) tasks.
Both companies position the deployment as part of a broader move toward AI-native discovery environments, emphasizing governance, traceability, and scalability. For Insilico, the integration supports its strategy of pairing internal drug development with deployable software and model offerings, while for Microsoft it serves as a reference implementation of Microsoft Discovery as an enterprise platform for coordinating domain-specific AI models, data, and compute.
Insilico reports that, across more than 20 internal programs initiated between 2021 and 2024, it has achieved preclinical candidate nomination in roughly 12 to 18 months on average, compared with industry timelines often cited at 2.5 to 4 years, with relatively limited synthesis and testing per program. Those figures are presented as context for how the company applies its AI stack in practice, though the Nach01–Microsoft Discovery integration itself is focused on workflow deployment.
Context
This Microsoft Discovery integration comes against a backdrop of heightened capital markets activity, partnering momentum, and pipeline progression at Insilico Medicine over the past two months:
- In November 2025, Insilico also expanded its relationship with Eli Lilly into a research and licensing collaboration carrying over $100 million in potential payments, extending a prior 2023 software licensing agreement and applying its Pharma.AI platform to jointly defined discovery targets.
- In late December 2025, the company raised more than US$290 million in its Hong Kong IPO, the city’s largest biotech listing of the year, becoming the first AI-driven drug discovery company to list on the Main Board under HKEX Chapter 8.05, a route that requires revenue or profit thresholds.
- More recently, in early January 2026, Insilico entered a multi-year oncology R&D agreement with Servier with a stated potential value of up to $888 million, under which Servier gains access to Insilico’s AI discovery stack and assumes downstream clinical and commercial responsibilities for selected candidates.
Operationally, Insilico has continued to advance AI-designed assets through the clinic. The company has reported progress on a gut-targeted PHD inhibitor for ulcerative colitis, developed using its generative AI platform and aimed at repairing the intestinal barrier, now advancing into Phase IIa studies. Separately, Insilico disclosed that it nominated a preclinical small-molecule candidate with Hisun eight months after launching their AI-driven discovery collaboration.
Insilico’s most advanced program, the TNIK inhibitor Rentosertib (ISM001-055), reported peer-reviewed Phase IIa clinical results published in Nature Medicine in June 2025, providing independent clinical validation of an AI-designed asset originating from its end-to-end discovery platform.
We track developments in AI-driven drug development and foundation models in our Where Tech Meets Bio weekly newsletter and deep dives on biomedical foundation models (“13 Foundation Models: Startups, Industry Updates and the Nobel Prize” and “17 More Biomedical Foundation Models: New Arrivals”) as well as agentic systems.
Topic: Tech Giants