Three Insights For Immuno-oncology Drug Discovery
Immunotherapies are hot property. Immuno-oncology is the crown jewel. But the road to riches, and more importantly cancer cures, is now crowded and full of potholes. Drug hunters need to look ahead, beyond the discovery process itself, to the reality of the many impediments that will confront drug candidates as they proceed towards the clinic in today's landscape. Here, I present three insights from current events, the third one taking a contrarian position.
Insight No. 1 is from John Carroll's Endpoints News coverage of the Cancer Research Institute's (CRI) latest "landscape" analysis in the December 2017 Annals of Oncology. There are 2,004 immuno-oncology agents crowding the pipeline, as inventoried in the graphic below. Just in 2017, for example, 469 new PD-1/L1 oncology studies were launched, requiring 52,000 patients to fully enroll them. There are already 164 PD-1/L1 drugs in the works, 50 in the clinic and 5 on the market. These alone have generated over 1,500 trials, before adding in the development burden of 344 cancer vaccines and 224 clinical-stage cell therapies.
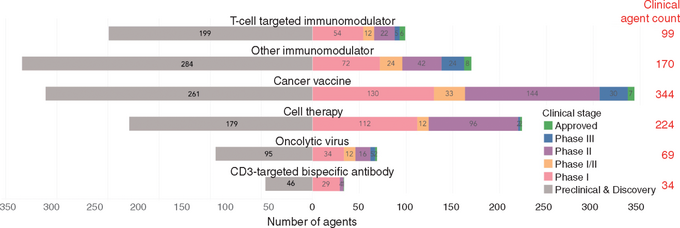
The sobering realization from reading these reports is that fanfare surrounding cancer immunotherapy presages an inevitable bust after such a boom. Blame the vagaries of clinical pharmacology, especially the requirements for PK/PD and what is rapidly becoming a structural inability to enroll enough patients. More numbers per trial will be needed to establish at a minimum a dose-response relationship, as well as a broad enough therapeutic index to guarantee safety. And the reality is that there aren't enough patients to go around.
In other words, the rules for efficacy demonstration in clinical trials will need major adjustment together with the overall design of those trials to incorporate features allowing more questions to be answered more efficiently in a single multicenter trial rather than in a patchwork of small studies, many of them investigator sponsored. The CRI report picks up on this fact. The premier, but seemingly ignored, advocate for upgrading to innovative umbrella, basket or platform trial designs is the FDA itself, with Janet Woodcock leading the charge for change in a July 2017 New England Journal of Medicine editorial review. A consensus along the same lines is also forming among oncology opinion leaders. It was highlighted with six articles in the September 2017 Focus Issue of Clinical Cancer Research (CCR).
Insight No. 2 is the need to develop and deploy a new generation of predictive preclinical models that can be translated into the clinic as molecular endpoints, and possibly, as surrogates for efficacy, especially given the FDA's recent acceptance of a molecular indication in an NDA. Elie Dolgin offered this perspective in a compelling "News & Analysis" piece for Nature Reviews Drug Discovery last December, with recommendations on how to better capture the benefits of checkpoint inhibitors, oncolytic viruses and modified T cell therapies.
Now, in 2017, enter PACT, the Partnership for Accelerating Cancer Therapies, an NIH led precompetitive consortium with 11 leading biopharmaceutical companies and the FDA as an active adviser. Armed with 215 million dollars, and possibly more as the corporate partners promote internal programs, "PACT will initially focus on efforts to identify, develop and validate robust biomarkers — standardized biological markers of disease and treatment response — to advance new immunotherapy treatments that harness the immune system to attack cancer." The pharma heavy hitters here, AbbVie, Amgen, Boehringer Ingelheim, BMS, Celgene, Genentech (Roche),Gilead, GSK, Janssen (J&J), Novartis and Pfizer, will work together under the NIH/FDA umbrella to facilitate systematic and uniform clinical testing of biomarkers explicitly for mechanisms of response and resistance to cancer therapy.
They will also seek to "integrate immune and other related oncology biomarkers into clinical trials by defining a set of standardized biomarkers to be tested across a variety of studies. This approach will allow for consistent generation of data, uniform and harmonized assays to support data reproducibility, comparability of data across trials, and discovery and validation of new biomarkers for immunotherapy and related combinations."
So here is the missing translational link to be watched, described further by Janice Mehnert and colleagues, writing on the challenge for development of valuable immuno-oncology biomarkers in the above-referenced CCR Focus Issue. Meeting this challenge, hand in hand with the discovery process itself, will prove indispensable in sustaining immuno-oncology as a viable R&D enterprise.
Insight No. 3 follows the received wisdom from many a research manager in the dictum "when in doubt, pull out." Getting into immuno-oncology in the midst of an apparent clinical trial log jam over hundreds of as yet unproven agents might not prove fruitful. A more tractable, less well known space, with defined target, efficacy models, biomarkers and clinical endpoints comprises immunotherapies focused on GPCR's, including many that have not been successfully "drugged" with the otherwise traditional small molecule effectors.
The field looks to be open. Orphan diseases and underserved conditions driven by GPCRs deserve attention and can also command marketplace premiums to justify discovery and development efforts otherwise wasted by late entrants into the immuno-oncology party. Want ideas? See Hutchings et al. and Hauser et al., both with relevant summaries in 2017 Nature Reviews Drug Discovery. With luck, there may be an unexpected and bonus therapy play here, since GPCRs are thought to be emerging anti-cancer targets from the summary evidence presented, also in 2017, by Gutierrez and McDonald in the journal Cellular Signaling.