Synchron Becomes First to Achieve Native Thought-Control of Apple iPad Using Brain-Computer Interface
The New York-based medtech Synchron, a brain-computer interface (BCI) company backed by DARPA, Gates Frontier, and Bezos Expeditions, has become the first to publicly demonstrate native thought-based control of an Apple iPad. The demonstration, featuring a participant with ALS, marks a significant milestone in both assistive neurotechnology and human-device integration
The system leverages Synchron’s Stentrode, a minimally invasive neural interface implanted via catheter into blood vessels near the motor cortex. Unlike systems that require open-brain surgery, the Stentrode captures motor intention signals from within the vasculature, translating them into digital commands in real time.
The breakthrough is enabled by Apple’s new Brain-Computer Interface Human Interface Device (BCI HID) protocol, released in May. This protocol allows BCIs to send input signals to Apple operating systems—such as iPadOS—natively, without middleware. It also enables closed-loop communication: the iPad shares live screen context with the BCI decoder, which improves signal interpretation and interaction accuracy.
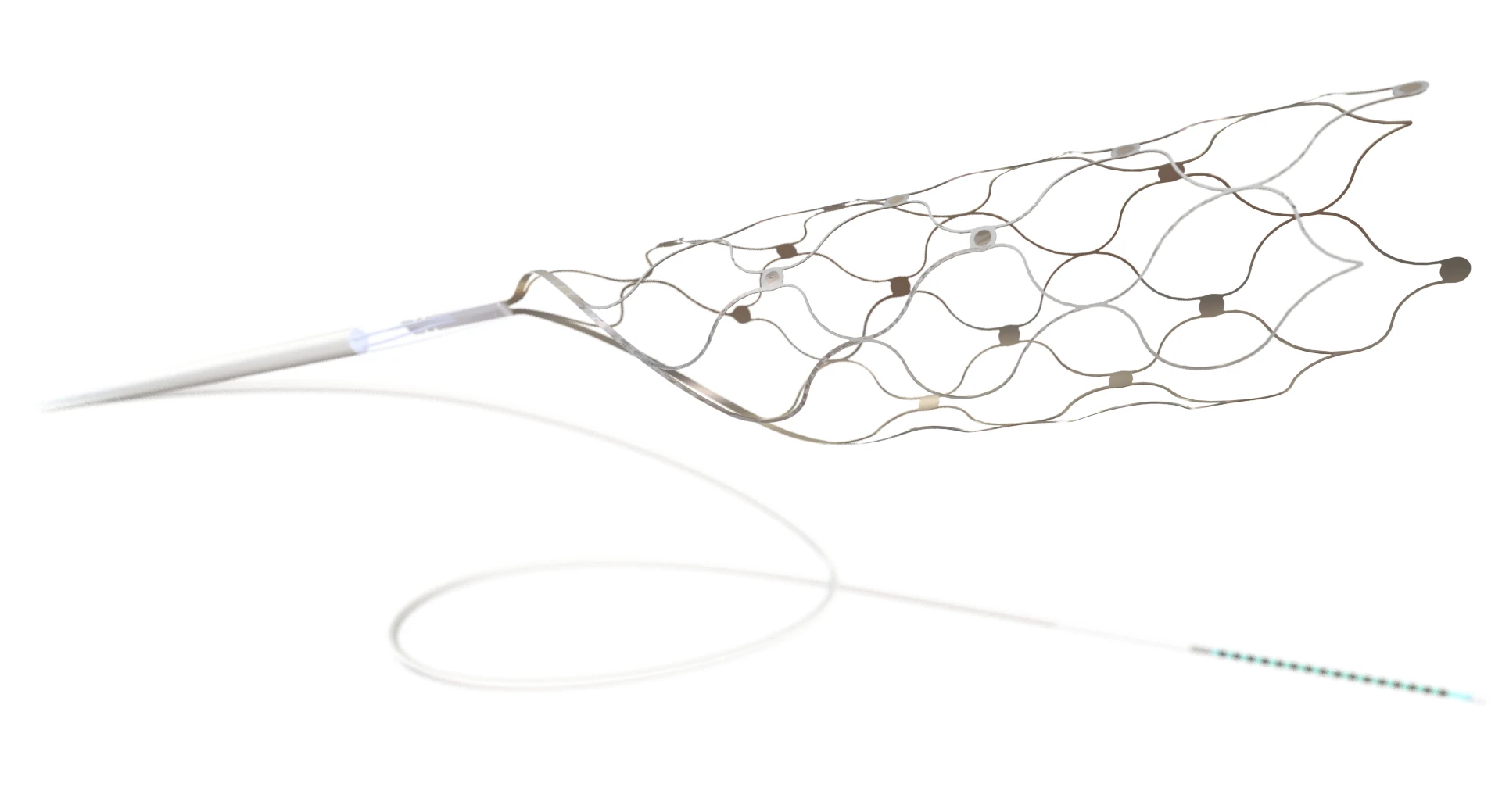
Image credit: Synchron
The demonstration features Mark, a real person living with ALS and a participant in Synchron’s ongoing COMMAND trial, using thought alone to navigate the iPad’s home screen, open applications, and compose messages. The system uses Apple’s built-in Switch Control accessibility feature to translate brain signals into UI actions.
Technical Significance of Synchrone’s BCI Tech
Synchron was founded in 2016 and is headquartered in Brooklyn, New York. Its core technology, the Stentrode, is a stent-mounted electrode array implanted via the jugular vein into blood vessels near the motor cortex, enabling long-term neural signal acquisition without requiring open-brain surgery.
This endovascular approach differentiates Synchron from competitors like Neuralink, which rely on more invasive craniotomy procedures. Unlike Neuralink’s fully implantable system, Synchron’s approach separates signal acquisition (implanted) from signal decoding (external), reducing surgical risk and increasing near-term scalability.
Interestingly, Synchron’s early development of the Stentrode began in Australia, led by neurologist Thomas Oxley and biomedical engineer Nicholas Opie within the University of Melbourne’s Vascular Bionics Lab.
The company launched its first feasibility trial, known as the SWITCH trial, in May 2019 at Royal Melbourne Hospital, enrolling four participants with ALS and other paralysis conditions to assess safety, signal stability, and usability of the Stentrode system.
Across a 12-month follow‑up, all four individuals performed real-world digital tasks, including emailing, online banking, shopping, and messaging, using thought-derived neural signals via an external decoder, with no serious device-related adverse events reported.
These successful outcomes supported the granting of an FDA Breakthrough Device designation in August 2020.
In 2024, the company demonstrated successful thought-based control of Amazon Alexa.
A Broader Conversation about BCI’s Future
This achievement comes at a time when brain-computer interface technologies are facing intensifying ethical and regulatory scrutiny.
Legal scholars, policymakers, and advocacy groups have raised concerns about neural data privacy, cognitive autonomy, and the adequacy of existing medical device regulations. In 2024, U.S. senators formally requested the Federal Trade Commission investigate how BCI companies handle neural data, warning that such information can reveal sensitive aspects of personality, intention, and mental health.
States like California and Colorado have already moved to classify brain-derived signals as sensitive personal data, requiring higher standards for consent and protection. Internationally, legal experts have called for coordinated governance frameworks to address emerging issues around mental privacy and discrimination.
In a 2024 interview I conducted with Dr. Brian Jamieson, former NASA engineer and current CTO of Diagnostic Biochips – another brain computer interface company, we discussed the implications of invasive versus non-invasive BCI technologies, including long-term viability, patient safety, and data governance.
Jamieson noted that while Neuralink has accelerated public attention, the real progress lies in technologies that prioritize clinical utility and scalability, particularly in contexts like drug discovery, neuromodulation, and assistive communication.
As the number of BCI users—currently under 100 globally—expands, so do questions of data ownership, cognitive privacy, and consent.
Nolan Arbaugh, Neuralink’s first human participant, recently raised concerns about the identity-level sensitivity of neural data. Legal experts have similarly highlighted the limitations of current oversight.
Daryl Lim, Associate Dean at Penn State’s ICDS, noted to The Debrief that the FDA alone is insufficient to regulate the full scope of risks posed by BCIs, and advocated for a coordinated supervisory body involving the FDA, FTC, HHS, NIST, and civil rights agencies
While the ethical and even philosophical debate is heating up, companies are racing to advance their BCI devices to grab the share of the future markets. Synchron is currently rolling out its BCI HID system to additional clinical participants, with plans for expanded access depending on trial outcomes. If successful, it would be the first BCI platform integrated into mainstream consumer hardware without requiring surgical craniotomy.
Topic: Next-Gen Tools