Strand Therapeutics Secures $153 Million for Programmable mRNA Cancer Therapies
Strand Therapeutics has closed a $153 million Series B financing led by Swedish investment firm Kinnevik, joined by Regeneron Ventures, ICONIQ, Amgen Ventures, Alderline Group, JIC-VGI, LG Technology Ventures, and Gradiant Corporation. Existing investors FPV Ventures, Playground Global, Eli Lilly, ANRI, and Potentum also participated. The round brings the company’s total funding to over $250 million and an estimated valuation of $550 million, up from $359 million in late 2024, according to PitchBook. Kinnevik’s Ala Alenazi, Ph.D., will join Strand’s board of directors.
The capital will support expansion of Strand’s lead candidate, STX-001, is a self-replicating mRNA therapy designed to express interleukin-12 (IL-12) locally within tumors, designed to reprogram the tumor microenvironment and trigger systemic immune responses. In an ongoing first-in-human Phase 1 trial in patients with advanced solid tumors, STX-001 has shown a favorable safety profile at doses up to 300 µg and preliminary anti-tumor activity, including confirmed complete and partial RECIST responses, prolonged disease stabilization, and evidence of immune activation in non-injected lesions. STX-001 uses synthetic mRNA genetic circuits for localized and prolonged therapeutic activity while aiming to limit systemic toxicity.
Data presented at the 2025 ASCO Annual Meeting indicated dose-dependent increases in plasma IL-12 and IFN-γ levels, along with immune cell infiltration in tumors. The trial has enrolled 22 patients with checkpoint inhibitor–refractory cancers across sites in the United States and Australia. Dose expansion is ongoing, with Phase 2 monotherapy trials and combination studies with checkpoint inhibitors planned.
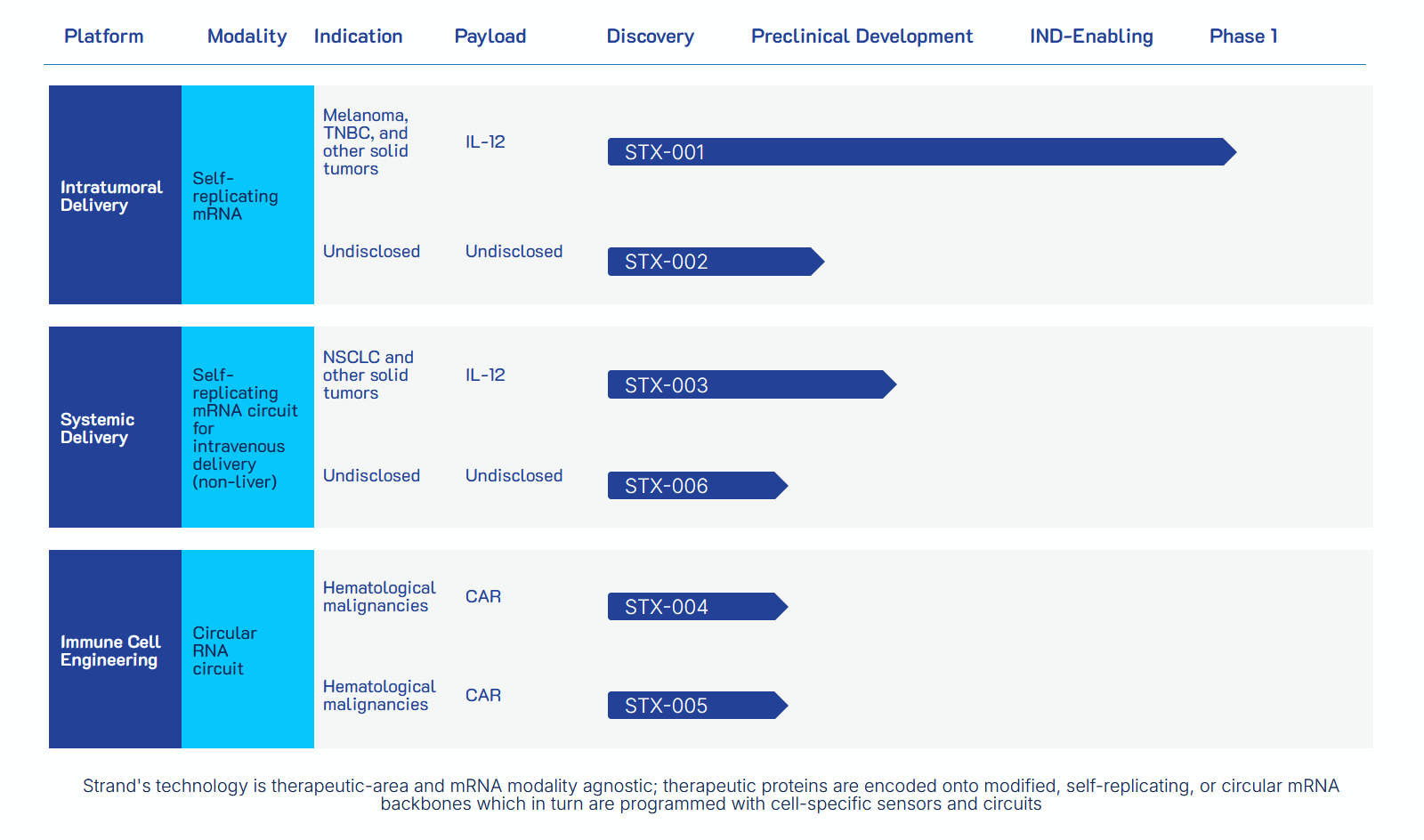
Strand's pipeline, August 2025
In addition to STX-001, Strand is developing STX-003, a systemically administered tumor-targeting mRNA therapy programmed to avoid off-target delivery, including to the liver. Preclinical results presented at the 2025 AACR and ASGCT meetings showed targeted IL-12 expression and anti-tumor activity with a favorable safety profile. Clinical entry for STX-003 is planned for 2026.
IL-12, the payload in both STX-001 and STX-003, is a pro-inflammatory cytokine known for stimulating anti-tumor immunity, but its systemic use has historically been limited by toxicity. Strand’s localized expression strategy seeks to retain efficacy while minimizing systemic side effects.
Founded in 2017 by Jake Becraft, Ph.D., Tasuku Kitada, Ph.D., and Ron Weiss, Ph.D., as a spinout from MIT, Strand Therapeutics applies synthetic biology to mRNA design, integrating genetic logic circuits for controlled therapeutic expression. The company aims to expand its approach to additional cancer types and other serious diseases, with a goal to bring its first therapy to market by 2030.
Topic: Biotech Ventures