Servier Signs €1B-Plus Multi-Target AI Drug Discovery Partnership With Iktos
Iktos and Servier have entered a multi-year, multi-target research collaboration under which Iktos will apply its AI-driven molecular design and automated synthesis platform to discover small-molecule drug candidates, while Servier will lead biological evaluation and downstream development. The agreement spans oncology and neurology programs and carries a stated potential value exceeding €1 billion across upfront payments, research funding, and milestones.
Under the collaboration, Iktos will use its generative AI and AI-orchestrated robotics infrastructure to design, synthesize, and optimize compounds against multiple undisclosed targets. Servier will assess these molecules using its internal biological assays and take responsibility for preclinical and clinical development of selected candidates. Financial terms beyond the aggregate deal ceiling were not disclosed.
Servier’s research leadership framed the partnership as part of its broader R&D strategy focused on alternative approaches to small-molecule discovery, with an emphasis on improving target understanding and candidate quality through external technology platforms.
Iktos Drug Discovery Platform Overview
Iktos operates an integrated drug discovery platform built around a fully digitized design-make-test-analyse workflow, combining multiple AI systems with automated chemistry and biology infrastructure. The platform is designed to couple in silico molecular design with rapid in vitro validation, allowing continuous feedback between computation, synthesis, and biological testing.
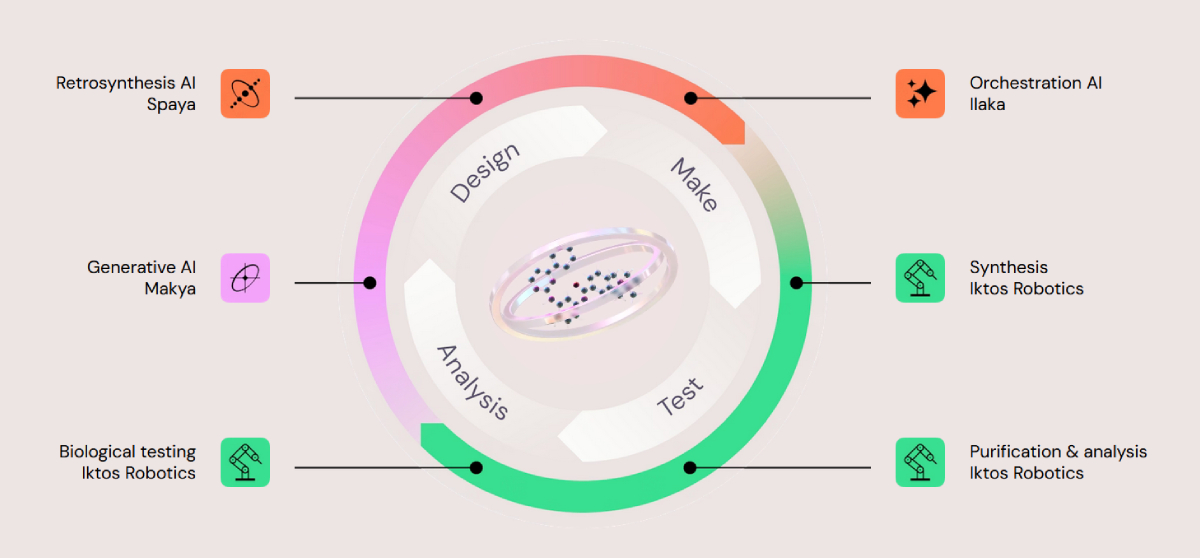
Iktos tech across DMTA cycle; Source: Iktos
At a technical level, the platform consists of several coordinated components:
- Makya, a generative AI system used to design small-molecule structures in silico based on project-specific objectives and constraints.
- Spaya, an AI-driven retrosynthesis engine that proposes synthetic routes compatible with automated chemistry systems.
- Ilaka, an orchestration layer that manages the end-to-end workflow, including material sourcing, experiment scheduling, and coordination of robotic synthesis and testing.
- Iktos Robotics, which integrates Chemspeed-based automated chemistry for parallel synthesis, alongside robotic purification, analytical characterization, and biological testing.
The robotic chemistry setup is reported to support on the order of 100 parallel reactions per day across more than 50 named reaction types. On the biology side, the platform incorporates high-throughput phenotypic assays, including cell-based readouts designed to generate large volumes of structured experimental data.
Founded in 2016, Iktos develops generative molecular design software coupled with automated chemical synthesis and biological testing. Iktos says it has worked on over 60 projects to date and is advancing its own pipeline in oncology, obesity and metabolism, and inflammatory and autoimmune diseases. Its most recent equity financing was a €15.5 million Series A round closed in September 2023. Subsequent platform expansion included the 2024 acquisition of Synsight to add high-content cellular imaging capabilities and a 2025 European Innovation Council Accelerator grant supporting further integration of AI and robotics within its discovery stack.
The Iktos agreement lands days after Servier’s separate, multi-year oncology R&D deal with Insilico Medicine that carried a stated potential value of up to $888 million and similarly gives Servier access to an external AI discovery stack with a handoff into Servier-led development. This pattern fits a wider 2025-early 2026 backdrop in which large pharma has been leaning harder on external dealmaking to refresh pipelines ahead of the patent cliff.
Some analysts have projected that roughly $40-45 billion in branded revenue could face generic competition each year over the next five years, creating sustained pressure to replace multiple high-revenue assets annually. In that environment, AI has increasingly appeared in collaborations where partners contribute model-guided chemistry and faster design–make–test loops, while pharma retains responsibility for biological evaluation and downstream preclinical, clinical, and commercial execution.
Topic: Industry Movers