Scribe to Start First Human Trial of One-Time CRISPR Therapy for Cardiovascular Disease
Scribe Therapeutics is preparing to enter the clinic in mid-2026 with Phase 1 studies of STX-1150, a one-time CRISPR-based therapy designed to silence genes linked to high cholesterol without cutting DNA. The therapy aims to durably suppress LDL cholesterol levels in patients with hypercholesterolemia, a major risk factor for atherosclerotic cardiovascular disease (ASCVD).
This is meant as a potential alternative to current cholesterol-lowering regimens that need lifelong dosing and suffer from poor patient adherence. The company also released a preprint detailing a newly engineered mechanism that adds a built-in safety control to their CRISPR platform.
If successful, it could mark the first clinical application of allosterically regulated CRISPR epigenetic silencing, offering an alternative to existing cholesterol drugs through a single administration with lasting impact.
See also: AI and CRISPR—converging revolutions in biomedicine?
About STX-1150
STX-1150 is based on Scribe’s proprietary Epigenetic Long-Term X-Repressor (ELXR) platform. Instead of using conventional CRISPR to cut DNA, ELXR uses a disabled version of the CasX enzyme—engineered to home in on a gene target without altering its sequence. It is fused to proteins that chemically modify nearby DNA and histones (the proteins that package DNA) to silence gene expression. Specifically, STX-1150 is designed to silence PCSK9, a gene that increases “bad” LDL cholesterol by degrading the liver receptors responsible for clearing it from the blood.
PCSK9 is a well-validated target: people who naturally carry loss-of-function variants in the gene exhibit lifelong low LDL levels and are protected against coronary disease without evident adverse effects. According to JACC’s latest report, existing PCSK9 inhibitors, including monoclonal antibodies and small interfering RNAs (siRNAs), must be taken repeatedly (ranging from biweekly to every six months) and adherence remains low, with only 1% of eligible patients currently receiving them. STX-1150 is intended to provide long-lasting suppression from a single dose.
In preclinical studies cited by Scribe, in non-human primates, a single low-dose lipid nanoparticle injection of STX-1150 reduced LDL cholesterol levels by more than 50%, with effects lasting 18 months and still ongoing. The therapy was well tolerated and did not cause significant liver toxicity.
Broader Cardiovascular Strategy
Beyond PCSK9, Scribe is building a pipeline targeting the other two key lipid drivers of ASCVD—lipoprotein(a) [Lp(a)] and triglycerides. Together with LDL-C, these three biomarkers are known contributors to plaque buildup in arteries, which can lead to heart attacks and strokes. Scribe’s broader approach is applying genetic tools not just for rare diseases, but for highly prevalent, chronic conditions with well-defined molecular drivers.
The company’s long-term goal is to use engineered CRISPR technologies to emulate the protective effects of beneficial human genetic variants, offering durable risk reduction from a single intervention. Ongoing strategic partnerships with Sanofi and Eli Lilly have supported the development of this platform and its transition to the clinic.
A New Control Layer for CRISPR Precision
Alongside the clinical announcement, Scribe published a preprint describing the molecular design underlying ELXR. The platform incorporates a synthetic control mechanism that mimics the body’s natural way of regulating DNA-modifying enzymes.
Normally, enzymes like DNMT3A (which add methylation marks to DNA to silence genes) are held in an inactive state and only activated when they encounter the right signals in the cell. Scribe’s ELXR integrates this autoinhibitory domain into its CRISPR construct, allowing the system to remain inactive until specific chromatin conditions are met at the target site.
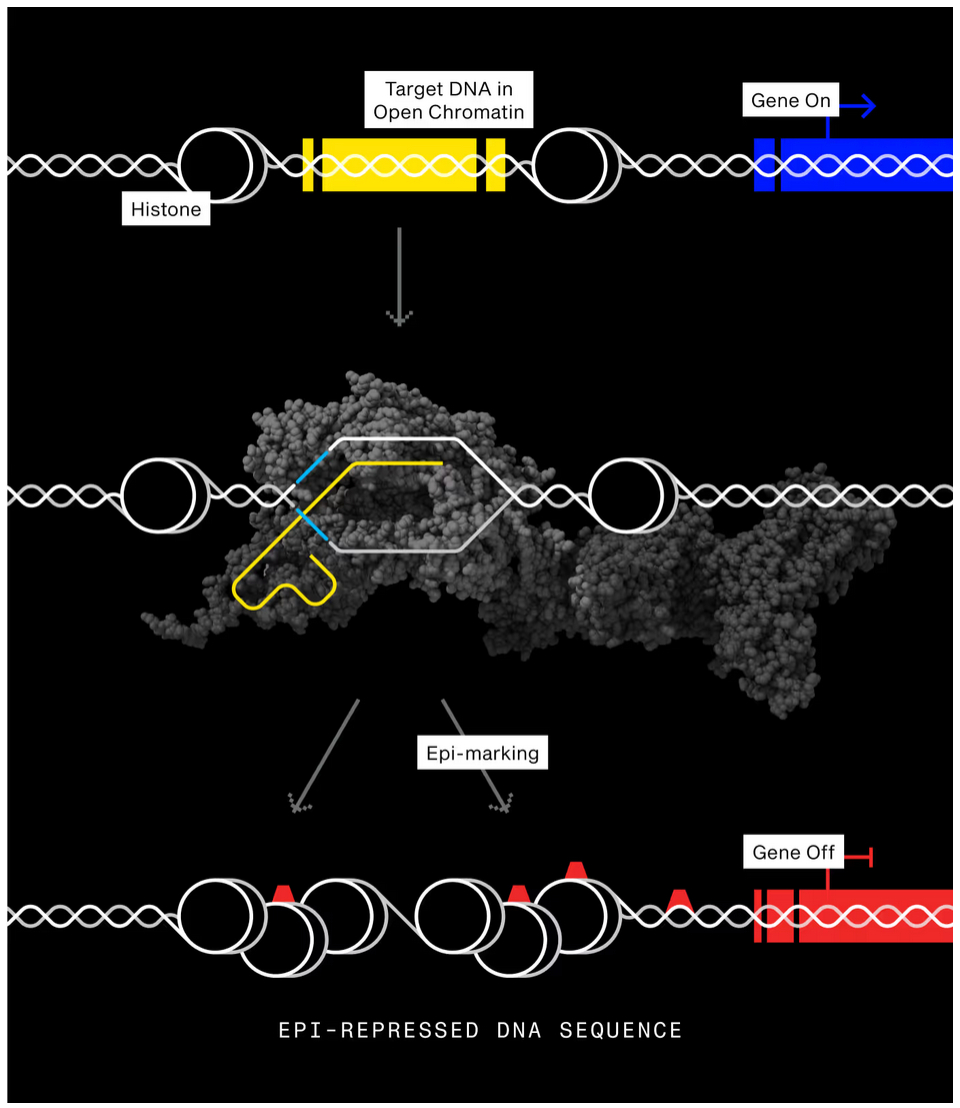
Image credit: Epigenetic Long-Term X-Repressor (ELXR), Scribe Therapeutics
This multi-step gating process requires both correct DNA matching (via the CRISPR guide RNA) and the right chromatin context, significantly reducing off-target effects. Transcriptome-wide profiling in the study showed that the allosteric ELXR cut down unintended gene expression changes by 10- to 100-fold compared to conventional constructs. This added control mechanism makes the therapy potentially safer and more precise, especially for preventive or long-term applications.
This announcement was part of Scribe’s presentation at the 44th Annual J.P. Morgan Healthcare Conference—see our weekly recap for a full overview of key developments from the conference, 2026 biotech and clinical AI reports, and more.
To learn more about how computational approaches are being used to expand the capabilities of gene editing technologies, see our deep dive, 12 Startups Applying AI to Gene Editing.
Topic: Next-Gen Tools