Scribe and Elli Lilly Reach Second Milestone in In Vivo CRISPR Collaboration
Scribe Therapeutics reported a second milestone, achieved with the help of Scribe’s proprietary X-Editor (XE) platform, in its multi-program collaboration with Eli Lilly focused on in vivo CRISPR-based therapies for neurological and neuromuscular disorders. The milestone advances one of the joint targets under the 2023 agreement and maintains momentum in Scribe’s broader genetic medicine pipeline, which also includes internal cardiometabolic programs.
Scribe, based in Alameda, California, was co-founded by CRISPR-pioneer Dr. Jennifer Doudna, and focuses on in vivo gene editing applications across prevalent diseases. In addition to Lilly, the company has established collaborations with Sanofi to expand the application of its engineered CRISPR systems.
The companies did not disclose the specific target or the financial value of the milestone. Under the 2023 collaboration terms, Scribe is eligible for more than $1.5 billion in aggregate milestone payments across programs, in addition to low-double-digit royalties on potential commercial sales. The partners previously reported an initial milestone achievement and presented joint data at the American Society of Gene and Cell Therapy meeting in 2025.
About X-Editor (XE)
The collaboration centers on Scribe’s engineered CasX nuclease, branded as X-Editor. X-Editor is Scribe’s CRISPR-based gene editing platform designed to make precise changes to DNA inside cells. At its core is an engineered enzyme called CasX, which acts like molecular scissors that can be directed to a specific genetic sequence. Once there, it cuts the DNA in a controlled way, allowing scientists to switch genes off, adjust how strongly they are expressed, remove sections, or insert new genetic material.
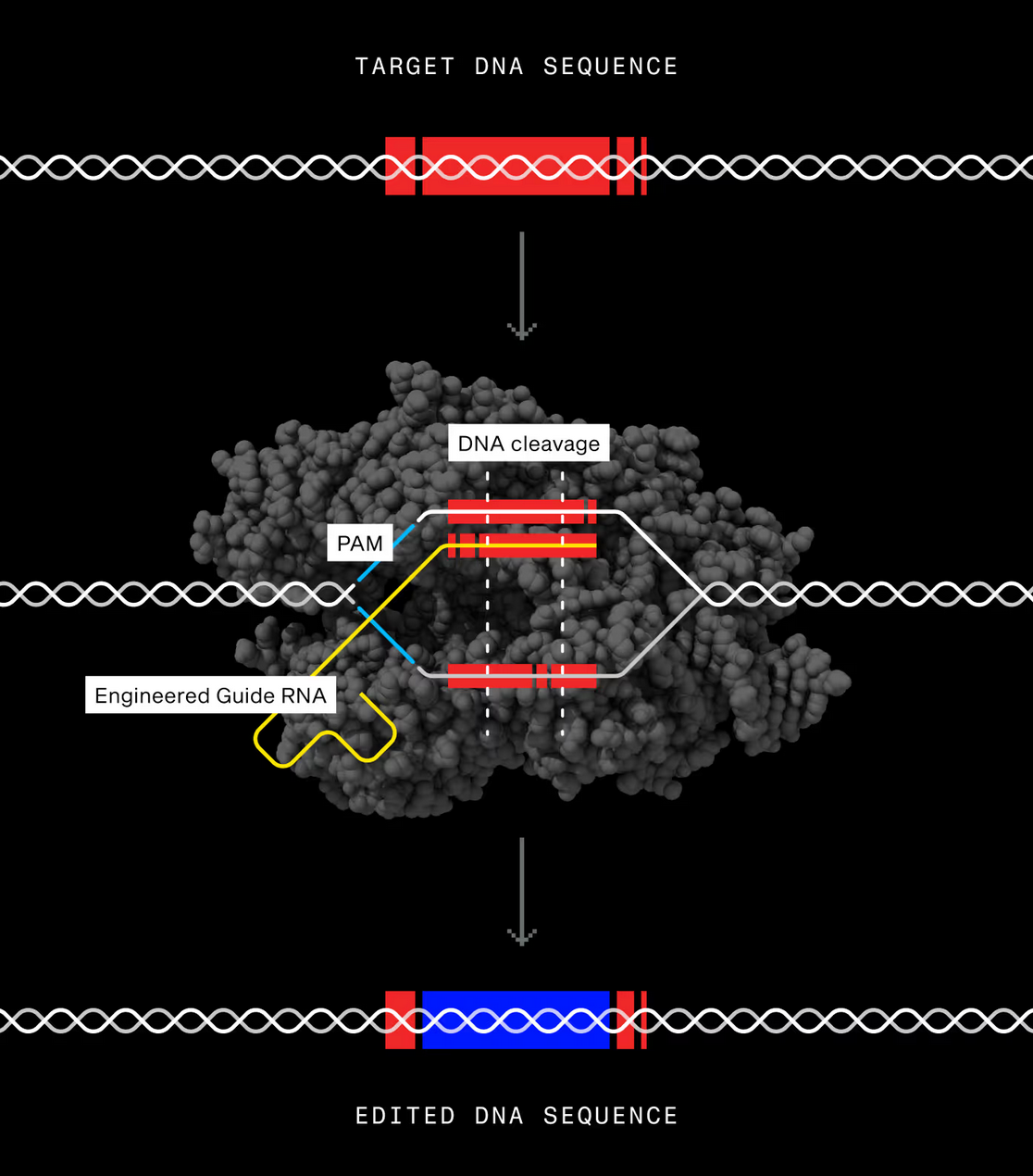
Image credit: X-Editor (XE), Scribe Therapeutics
Scribe reports that its modified version of CasX edits DNA far more efficiently than the naturally occurring form in laboratory cell studies, while maintaining accuracy at the intended target site. The enzyme is also relatively small, which may make it easier to deliver into tissues using standard gene therapy vectors. Together, these features are intended to support the development of one-time genetic treatments for diseases caused by defined DNA changes.
Broader Pipeline Context
Beyond the Lilly partnership, Scribe is advancing wholly owned cardiometabolic programs. Its lead candidate, STX-1150, is described as a liver-targeted therapy intended to epigenetically silence PCSK9, a gene that increases “bad” LDL cholesterol, without introducing permanent DNA sequence changes. The company has stated that it expects to initiate clinical development of STX-1150 in mid-2026 for hypercholesterolemia, and recently announced it would start Phase I human trials of STX-1150.
In that same announcement, Scribe published a preprint describing the molecular architecture behind its ELXR, a platform that includes an engineered control system designed to emulate the cell’s natural regulation of DNA-modifying enzymes.
Topic: Next-Gen Tools