Roche and Manifold Bio's up-to-$2B Deal to Develop Next-Gen Brain Shuttles for Neurological Diseases
Manifold Bio, a Boston-based platform therapeutics company, announced a strategic collaboration and license agreement with Roche to develop several next-generation blood-brain barrier (BBB) shuttles intended for neurological and neurodegenerative disease programs. The work will apply Manifold’s tissue-targeting shuttle portfolio and its mDesign AI-guided discovery engine to generate shuttle molecules that can move therapeutic payloads into the brain.
BBB shuttles are targeting modules that help large drugs like antibodies or RNA therapies cross the blood–brain barrier via receptor-mediated transport, increasing drug exposure inside the brain. They can turn systemically dosed biologics into viable CNS treatments, potentially lowering required doses and widening the range of neurological diseases that can be addressed.
Manifold’s approach is designed to measure thousands/up to millions of biologic variants directly in vivo, producing physiologically relevant data at scale. The platform screens for shuttles that traffic via different endothelial “portals” (receptors) and then pairs the best performers with various modalities to expand brain exposure and potentially improve dose/exposure trade-offs seen in first-generation BBB approaches.
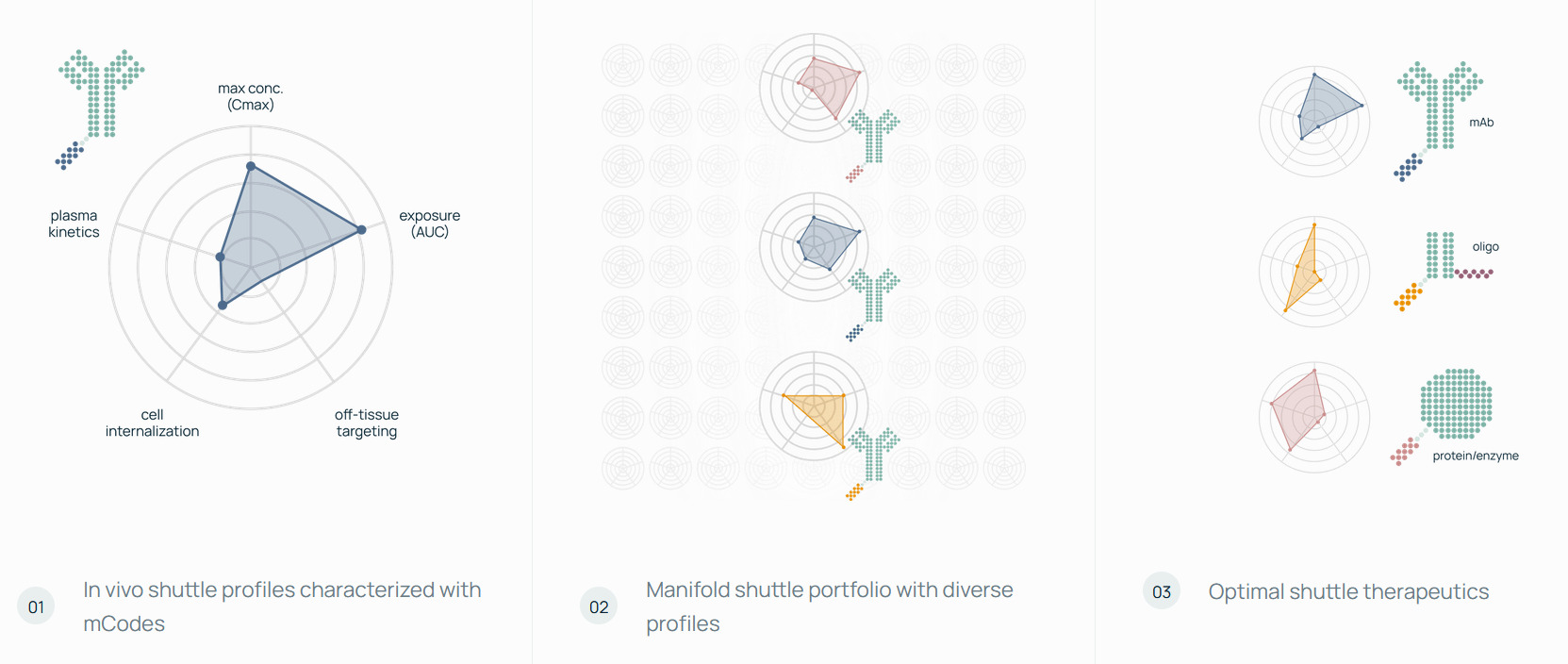
Image credit: Manifold Bio
AI Discovery Engine & Future In-Vivo World Model
Manifold Bio’s mDesign pairs AI-guided protein design with direct testing in living systems to tune pharmacokinetics, biodistribution, and other properties for tissue-specific delivery. A core component is mCodes (small peptide barcodes attached to each therapeutic and read via affinity capture and DNA sequencing) which enable quantifying hundreds of candidates across tissues in a single animal. The system reportedly reaches single-digit picomolar sensitivity, offers 1,000+ barcodes, and supports millions of protein–barcode combinations.
The mDesign stack uses structure-guided models for precise epitope targeting and zero-shot, controllable binder design, trained on massively multiplexed binding data from high-throughput synthesis and display; the longer-term goal is a predictive in-vivo world model that links molecular design to whole-body behavior and therapeutic outcomes.
The Deal
Under the deal, Manifold receives $55 million upfront and is eligible for research, preclinical, clinical, and commercial milestones that could exceed $2 billion, plus tiered royalties on sales. Manifold also holds an option to co-fund development of one product candidate in exchange for higher royalty participation and retains full rights to deploy its BBB shuttles on non-licensed targets in internal or future partnered programs.
The collaboration divides execution:
- Manifold will run research and discovery to identify BBB shuttles tailored to selected Roche payloads;
- Meanwhile, Roche leads subsequent preclinical development, clinical trials, and commercialization.
Roche brings longstanding BBB shuttle development experience and downstream capabilities to progress any selected shuttle-payload combinations into the clinic. If successful, the partnership could increase the range of biologics that reach the brain, opening options across multiple CNS indications.
Topic: Industry Movers