NIH Foundation Launches Industry-Backed Program to Advance Non-Animal Testing Methods Toward Regulatory Use
The Foundation for the National Institutes of Health (FNIH) has launched the Validation and Qualification Network (VQN), a multi-stakeholder initiative aimed at accelerating the regulatory acceptance of alternatives to animal testing in drug development.
Announced on July 24, the VQN is part of the NIH’s broader Complement Animal Research in Experimentation (Complement-ARIE) program, initiated in 2024 under former U.S. administration. It brings together dozens of partners, including U.S. and European regulators, major pharmaceutical companies like Sanofi, Novo Nordisk, and GSK, as well as CROs such as Charles River Laboratories.
Unlike traditional NIH grant models, the VQN will not fund projects upfront. Instead, it will select promising alternative method developers, then help refine their projects collaboratively. Within each project, pharma, regulators, and nonprofit partners will work jointly to define acceptable readouts and align technical parameters to ensure consistency and regulatory relevance, all while allowing developers to retain their core technology and intellectual property. The selected projects—four in this first round—will then be externally funded by network members. Submissions are being accepted on a rolling basis through December 31, 2025.
Projects must also demonstrate how they contribute to reducing, replacing, or refining the use of live animals (the "3Rs") in research. The VQN is especially interested in combinatorial projects—integrated approaches that combine multiple NAMs to achieve greater predictive power than current testing methods.
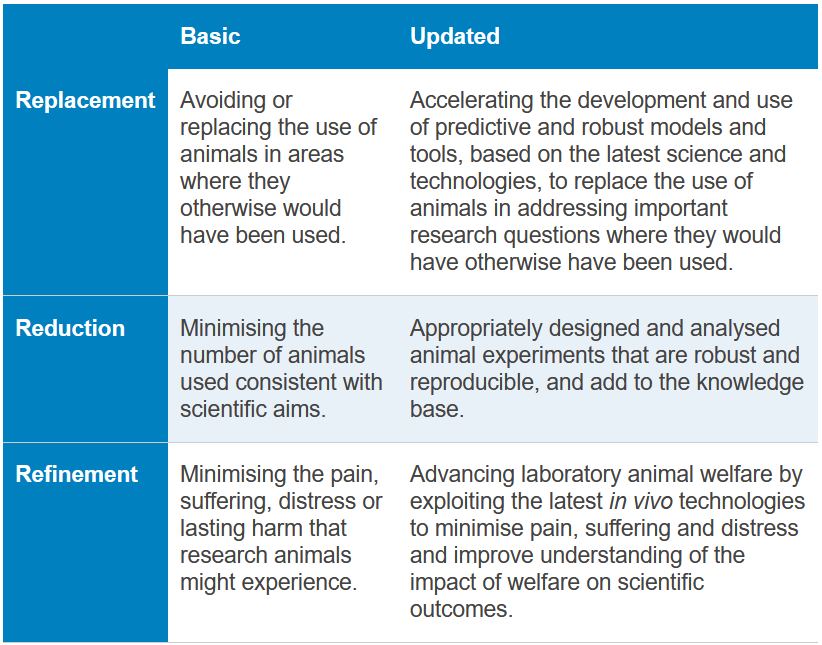
The 3Rs, The National Centre for the 3Rs
The VQN’s long-term goal is to build a central hub that helps new approach methodologies (NAMs) become validated tools for regulatory submissions. The effort follows increased federal interest in non-animal testing approaches under the current U.S. administration. In April 2025, the FDA announced plans to phase out animal testing for monoclonal antibody therapies and other drugs, and NIH launched the Office of Research Innovation, Validation and Application (ORIVA) to support the agency’s work on developing NAMs.
The VQN operates at the final stage of NAM development, concentrating on approaches nearing regulatory acceptance. These NAMs include AI-based computational toxicology models, human-derived organoid systems, and organ-on-a-chip platforms, which together offer mechanistic insights, faster turnaround, and greater relevance to human biology. Their implementation is already underway in investigational new drug (IND) applications, as part of a broader shift toward modernized, human-relevant testing that reduces costs, shortens development timelines, and minimizes animal use.
Though thousands of NAMs are already in development, most remain unapproved for regulatory use. VQN aims to fill this gap by adapting the model of the Biomarkers Consortium, which has supported over 40 projects since 2006. Managed by FNIH, the Consortium is a public-private partnership focused on validating and qualifying biomarkers and other drug development tools to support regulatory decision-making. It advances tools such as disease biomarkers and surrogate endpoints to enable innovation in drug development, preventive medicine, and diagnostics. Its efforts are funded by private-sector membership and involve collaboration with NIH, FDA, CMS, and over 70 industry and nonprofit partners. VQN draws from this collaborative, multi-stakeholder model to help bridge the translational gap and promote regulatory readiness of NAMs.
Charles River and Sanofi have already contributed modest financial support to the VQN, with plans to invest further in aligned projects. While stakeholders acknowledge that animal testing will not be entirely eliminated, the goal of the VQN is to strategically reduce its use where scientifically feasible and expand the use of scientifically validated alternatives in preclinical drug development.