New Neurodegenerative Disease Biotech Launches With $175M to Test Brain-Penetrant Antibody for Alzheimer's
Korsana Biosciences has launched with $175 million in combined seed and Series A financing to develop brain-penetrant antibody therapies for neurodegenerative diseases. The company’s main investigational drug, KRSA-028, is an antibody therapy designed to cross the protective blood-brain barrier by attaching to a naturally occurring protein on blood-vessel cells known as transferrin receptor 1.
The Waltham, Massachusetts-based company was founded in 2024 with a $25 million seed investment from Fairmount and Venrock Healthcare Capital Partners. In September 2025, it closed a $150 million Series A round co-led by Wellington Management and TCGX, with participation from J.P. Morgan Life Sciences Private Capital, Janus Henderson Investors, Sanofi Ventures, and Foresite Capital, among others.
It is led by Jonathan Violin, Ph.D., who previously served as founding CEO of Viridian Therapeutics, Dianthus Therapeutics, and Quellis Biosciences. The company represents the seventh launch based on assets discovered by Paragon Therapeutics, a biotechnology firm founded in 2021 that develops complex biologics for advancement through partner companies.
Korsana’s main experimental drug, KRSA-028, is a specially engineered “monoclonal antibody”—a lab-made protein that can recognize and bind to amyloid beta, a protein that forms sticky clumps in the brains of people with Alzheimer’s disease. Most existing Alzheimer’s antibodies have limited ability to reach the brain and act on these amyloid deposits.
The company’s experimental antibody was discovered together with Paragon Therapeutics and built on the company’s THETA platform, a method that uses two engineered features to help large therapeutic proteins reach the brain. One part of this method engages transferrin receptor 1 (TfR1), a protein on the surface of cells that form the blood-brain barrier. TfR1 normally helps bring iron into the brain by binding its natural partner, transferrin. In drug development, this receptor can be repurposed as a “shuttle” so that therapeutic antibodies bound to it are ferried across the barrier via a process called receptor-mediated transcytosis. Research in experimental models shows that attaching an antibody to a TfR1-binding component can significantly increase its delivery into the brain compared with the unmodified antibody.
The second engineered feature relates to Fc engineering. The Fc region is part of an antibody that can be modified to alter how the antibody behaves in the body, including how it is transported, how long it circulates, or how it interacts with immune cells. Adjusting the Fc region can change how strongly the antibody engages receptors like TfR1 and how it is sorted inside cells, with the goal of improving delivery into the brain.
According to the company, KRSA-028 is engineered to increase plaque removal while reducing the chance of ARIA and blood-related side effects, and it’s being formulated as an injection under the skin to make treatment simpler compared with intravenous infusions used in existing Alzheimer’s anti-amyloid therapies. These plaques are clusters of a protein called amyloid beta that tend to build up in people with Alzheimer’s disease and are thought to disrupt normal brain function.
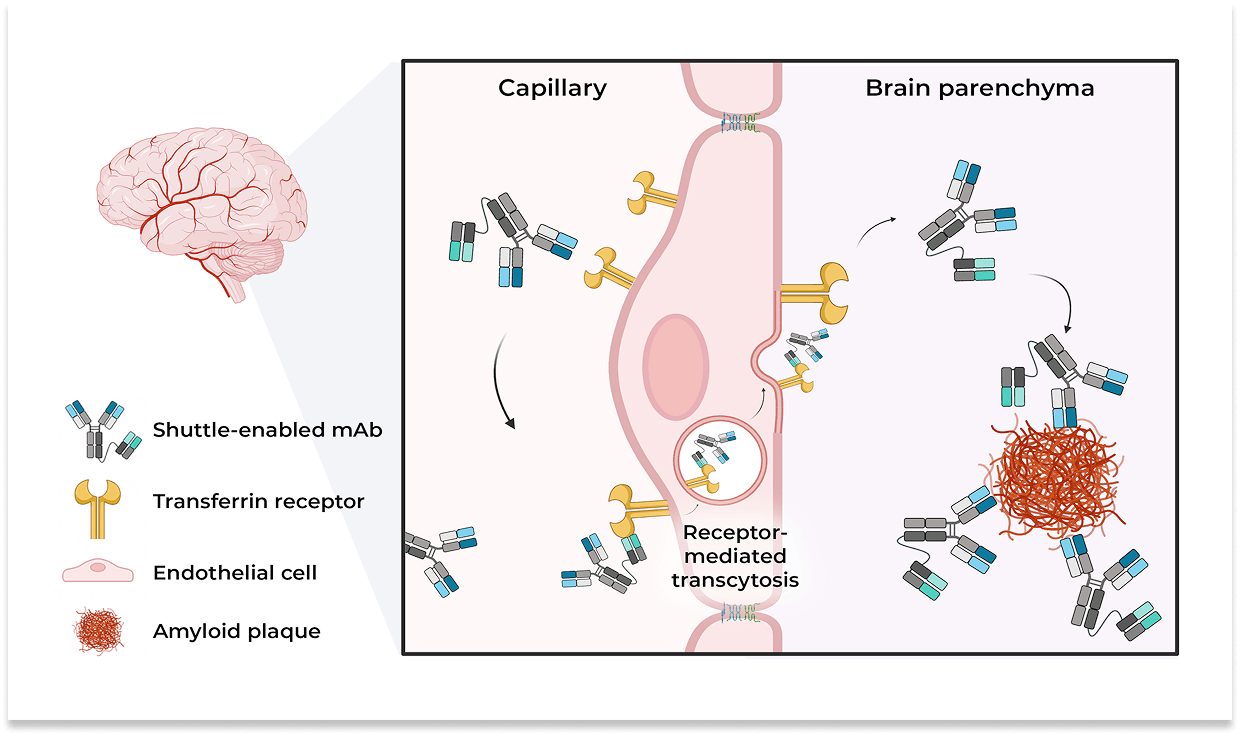
Image credit: Korsana Biosciences
In past anti-amyloid antibody therapies, some people developed amyloid-related imaging abnormalities (ARIA), which are changes seen on brain scans after treatment. ARIA can include swelling or small spots of bleeding in the brain. These abnormalities are linked with how the plaques are cleared and how the blood vessels respond to that process.
Initial clinical objectives include pharmacokinetic, central nervous system penetration, and safety data from healthy volunteers, anticipated in mid-2027. Proof-of-concept data evaluating amyloid plaque clearance in Alzheimer’s patients are expected by the end of 2027. The company states that its current financing is intended to support operations through these milestones and into 2028.
Topic: Next-Gen Tools