Insilico Medicine Launches AI Gym for LLMs and Secures FDA Clearance for AI-Designed NLRP3 Inhibitor
Just a few days after Insilico Medicine and Hygtia Therapeutics announced a $66 million co-development deal for ISM8969, the AI-designed drug has now cleared a key regulatory step to enter the clinic.
Additionally, Insilico has introduced Science MMAI Gym, a new domain-specific AI training framework aimed at upgrading general-purpose large language models (LLMs), such as ChatGPT, Claude, Gemini, among others, into domain-specialized engines suitable for pharmaceutical research and development. The platform is designed to correct performance gaps observed when LLMs are applied directly to biomedical and chemical tasks without domain conditioning.
The U.S. FDA has approved the investigational new drug application for ISM8969, allowing Phase I trials to begin in healthy volunteers. ISM8969 is an oral, brain-penetrant inhibitor of NLRP3, a protein involved in chronic inflammation linked to Parkinson’s disease, and was discovered and optimized using Insilico’s generative AI platform, Chemistry42.
The Gym forms part of Insilico’s broader roadmap toward what it terms Pharmaceutical Superintelligence (PSI). It includes two dedicated architecture tracks: Chemical Superintelligence (CSI) and Biology/Clinical Superintelligence (BSI).
LLMs are trained in the Gym using multi-task supervised fine-tuning (SFT), reinforcement fine-tuning (RFT), and proprietary scientific datasets. Reportedly, Insilico draws from over 4 million medicinal chemistry optimization chains, 100 million organic synthesis descriptions, and hundreds of thousands of molecular dynamics simulations.
Internal evaluations show marked performance gains. A previously baseline-level open-source LLM reportedly achieved state-of-the-art (SOTA) results on ADMET prediction and multiple optimization tasks within the MuMO-Instruct framework after CSI training. In the BSI track, models improved their F1 score on ClinBench and outperformed other LLMs on TargetBench in novel target identification, including across translational disease models.
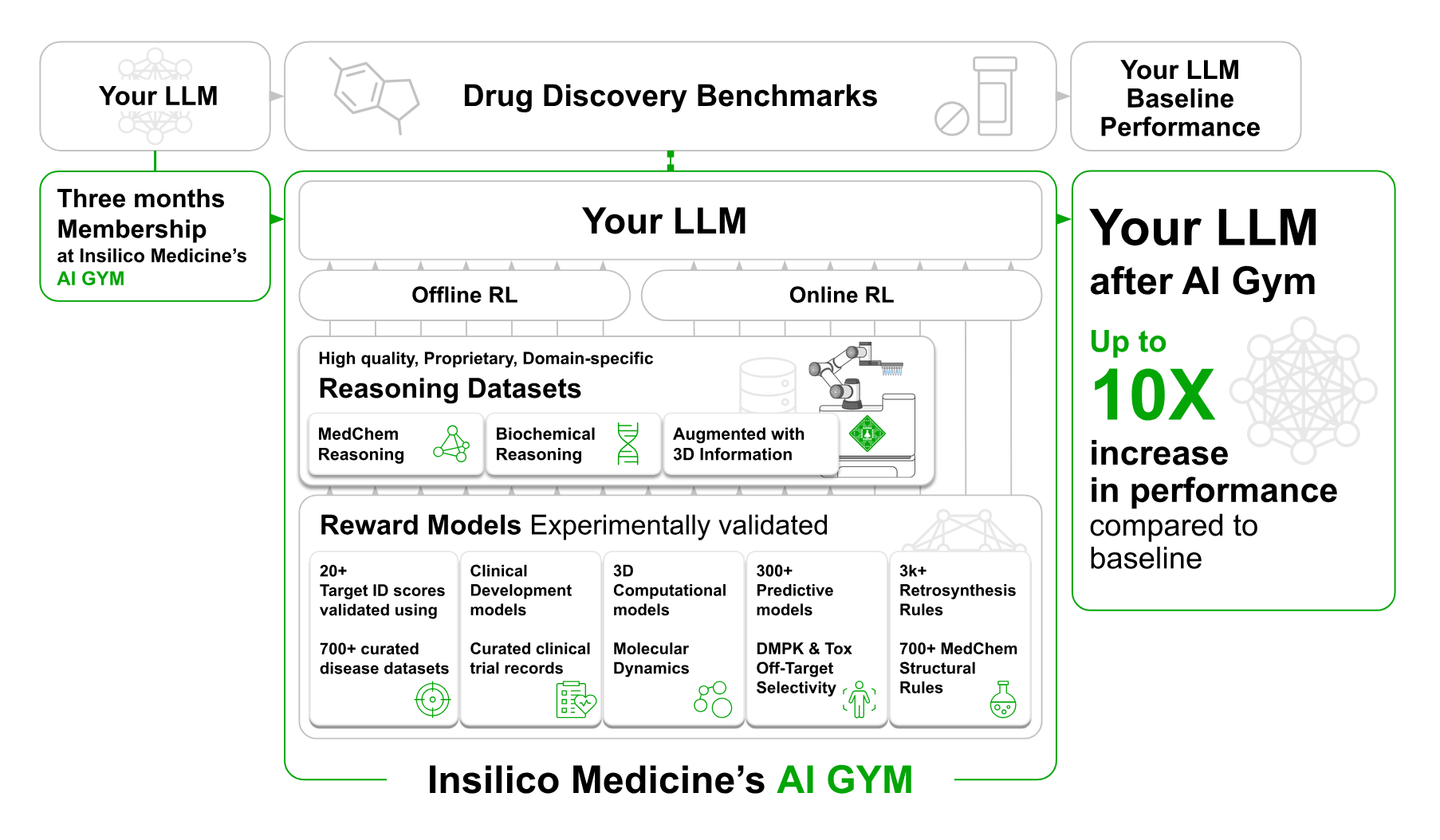
Image credit: Insilico Medicine
Training Structure and Availability
The architecture uses high-volume, experimentally grounded data to instill domain-specific reasoning. In chemistry (CSI), this includes retrosynthetic planning, reaction pathway prediction, and 3D structure-activity inference. In biology and clinical science (BSI), tasks involve omics-integrated disease modeling, clinical trial endpoint interpretation, and Phase 2 success prediction. Models are assessed using both public and proprietary benchmarks, such as TDC, TargetBench, and ClinBench, the latter focused on real-world trial prediction tasks.
Science MMAI Gym is available to external partners, biopharma companies, AI labs, and cloud vendors via a membership model. Engagements range from two-week CSI/BSI adaptation sprints to long-term PSI development programs. Participating partners provide their LLMs and receive enhanced models along with performance diagnostics and optional validation in Insilico’s automated wet-lab systems.
In 2025, Insilico has continued expanding its AI-driven drug discovery model through partnerships with major pharma companies, including a multi-program collaboration with Sanofi valued at up to $1.2 billion, a research and licensing deal with Eli Lilly, and a series of oncology agreements with Menarini Group. In late 2025, the company completed its IPO on the Hong Kong Stock Exchange, raising around US$290 million in the year’s largest biotech listing on that market.
Topic: AI in Bio