In an AI-Enabled ALS Study, Digital Twins Support Gene-Delivered Antibody Trial
In a collaboration that embeds AI directly into trial design and analysis to reduce statistical and operational risk, Unlearn will deploy patient-level AI-generated digital twins in VectorY Therapeutics’ PIONEER-ALS Phase 1/2 study. The digital twins will serve as external comparators in a single-arm trial of VTx-002, a vectorized antibody targeting TDP-43 pathology, a hallmark of amyotrophic lateral sclerosis (ALS). The approach is designed to strengthen exploratory analyses and reduce uncertainty in outcome interpretation in a disease area where placebo-controlled trials are often difficult to execute.
See also: Companies Applying AI to De-Risk Clinical Trials: 2026 Watchlist
VectorY is an Amsterdam-based biotech founded in 2020 that develops one-time gene-delivered antibody treatments for neurodegenerative diseases such as ALS, Huntington’s, and Parkinson’s. Its platform combines engineered antibodies that selectively bind harmful proteins, customized AAV delivery vehicles designed to reach the brain more effectively, and scalable manufacturing systems to produce these gene therapies at larger volumes and lower cost.
VectorY will use Unlearn’s ALS Digital Twin Generator, a machine learning model trained on historical, patient-level ALS clinical datasets. For each enrolled participant, the system generates an individualized prediction of expected disease trajectory under standard of care. These predictions function as participant-level external comparators and are incorporated into prespecified exploratory analyses.
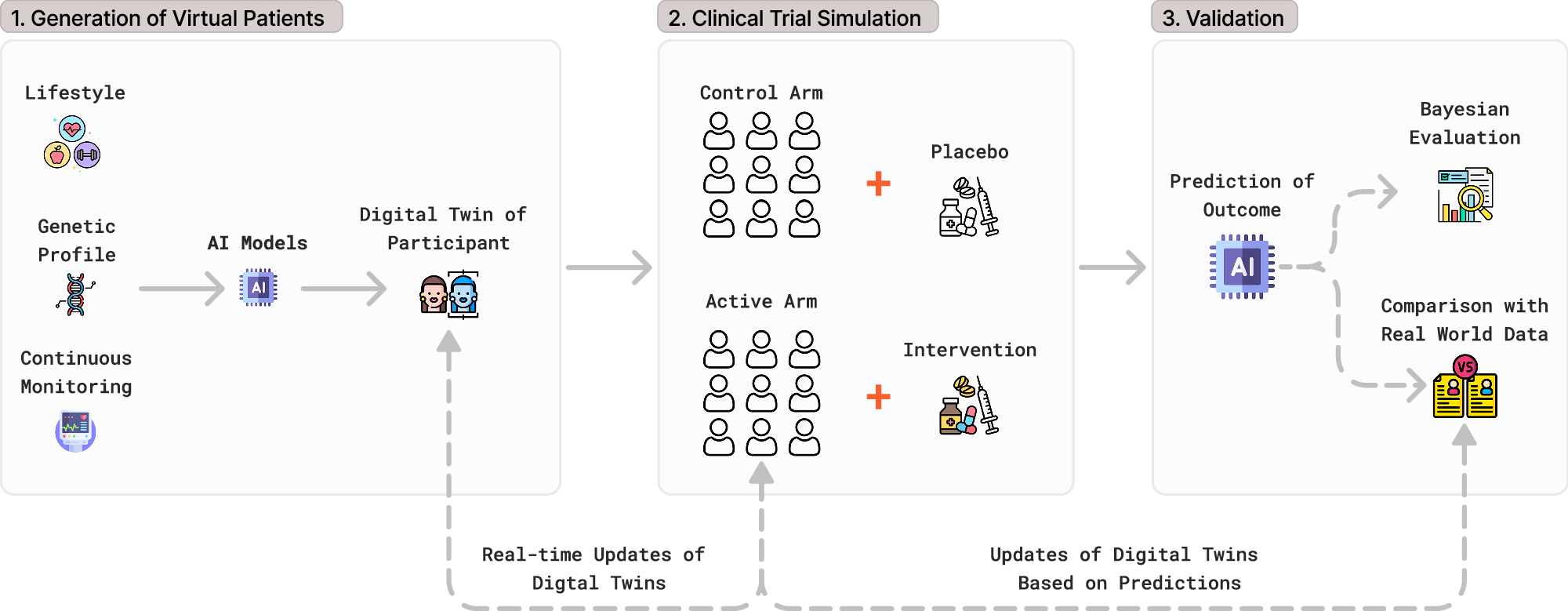
Image credit: Enhancing randomized clinical trials with digital twins, Akbarialiabad, H., Pasdar, A., Murrell, D.F. et al.
VectorY’s VTx-002 is not a conventional small molecule. It is a gene-delivered antibody therapy, combining an AAV vector with a genetic payload encoding a therapeutic antibody. This places it within the broader class of advanced therapeutic modalities, where the format of the medicine (viral delivery, genetic instruction, in vivo antibody production) is central to its mechanism.
For a broader analysis of how antibodies, nucleic acids, cell and gene therapies, and targeted protein degraders are reshaping pipeline value and deal flow, see our recent dive into New-Modality Drugs Behind Today’s Big Headlines.
ALS is a rapidly progressive neurodegenerative disease with a median survival of two to three years. In the US, roughly 5,000 new cases are diagnosed annually, with about 30,000 people living with the condition. High mortality and low prevalence make traditional placebo-controlled trials difficult, so sponsors often rely on single-arm studies, which complicate efficacy interpretation due to the absence of an internal control.
At the biological level, ALS is marked in most patients by misfolded TDP-43 protein that accumulates outside the cell nucleus as toxic clumps, while its normal nuclear function is lost. Vectorized antibody therapies aim to address this by using a viral delivery system to introduce genetic instructions into cells, enabling them to produce an antibody that binds and helps clear these toxic TDP-43 aggregates, with the goal of reducing cellular damage and restoring protein balance.
Unlearn states that it has engaged with regulators on its digital twin framework, including qualification with the European Medicines Agency and support from the US Food and Drug Administration, as part of broader efforts to apply AI-based external comparators in clinical development.
Topic: Next-Gen Tools