Iambic’s AI-Designed Cancer Drug Shows Early Clinical Activity
Iambic Therapeutics presented new data from its ongoing Phase 1/1b trial of IAM1363 at the 2025 ESMO Congress, showing anti-tumor activity in heavily pretreated patients with HER2-driven cancers, including those previously treated with T-DXd and tucatinib. The AI-designed small molecule demonstrated responses across HER2-wild-type and HER2-mutant tumors, including intracranial lesions.
IAM1363 is an irreversible, type II HER2-selective tyrosine kinase inhibitor with over 5,000-fold selectivity versus EGFR, designed for brain penetration and activity across multiple HER2 alterations. Partial responses were observed in 28% of patients (N=18) with measurable systemic disease and 33% (N=3) with measurable intracranial tumors. Activity was seen in tumor types with limited or no approved HER2 TKIs, including HER2-mutant renal cell carcinoma and HER2-amplified NSCLC and ovarian cancer.
The compound was developed using Iambic’s AI-driven discovery platform, which combines transformer-based models (e.g. Enchant, launched last October), structure prediction tools (e.g. NeuralPLexer), and integrated design-make-test cycles on a weekly cadence. IAM1363 progressed from discovery to first-in-human dosing in under two years.
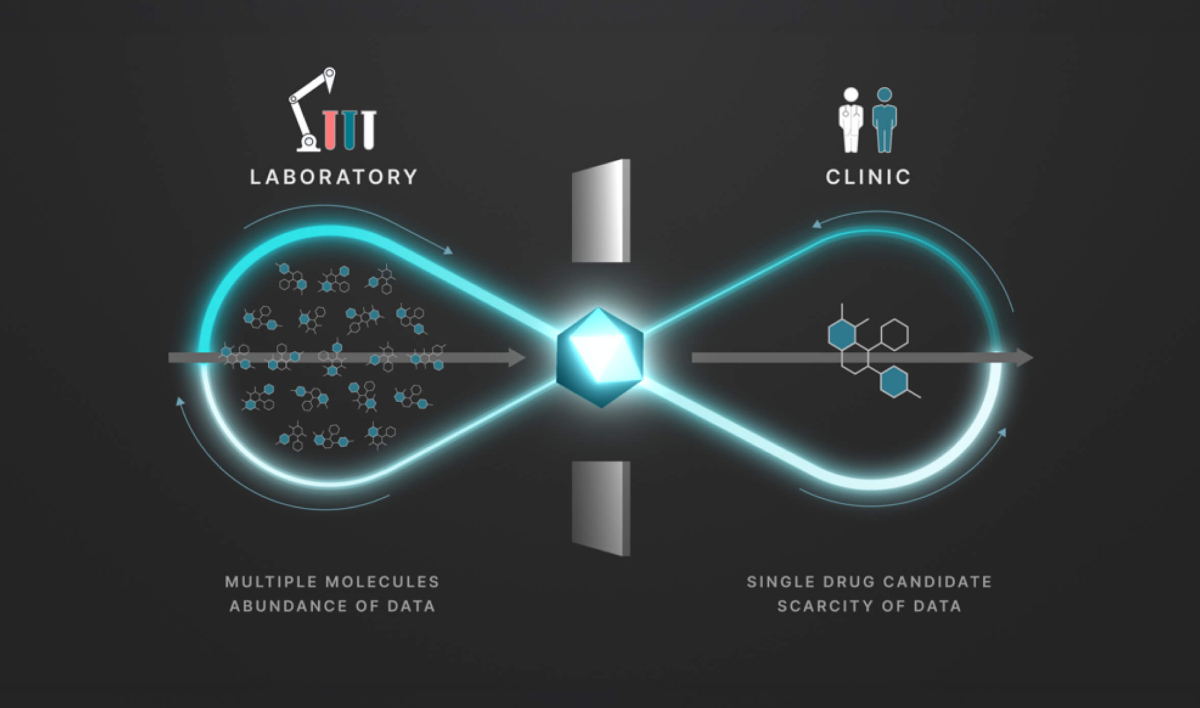
Iambic's Enchant model is said to predict clinical drug properties from limited clinical and rich lab data.
We profiled Iambic’s AI platform in our 2025 AI Drug Discovery report as a representative of the emerging AIDD archetype—combining generative design, structure prediction, and clinical property inference into an end-to-end, in silico pipeline. According to information provided by Iambic representatives at the time of writing the report, IAM1363 completed its IND-enabling phase in approximately eight months.
The first patient was dosed in March 2024, the company subsequently raised a $50 million Series B extension in June 2024, bringing its Series B total to over $150 million. The new funds are supporting expansion of Iambic’s internal pipeline and partnered oncology programs.
The Phase 1/1b study is an open-label, multi-center dose escalation and optimization trial evaluating IAM1363’s safety, pharmacokinetics, and preliminary efficacy. It is active in the U.S., recently opened in the EU, and is expected to expand to the UK and APAC in Q4 2025. Combination therapy cohorts are also planned.
Topic: AI in Bio