FDA Grants De Novo Authorization to ArteraAI’s Multimodal AI Prostate Cancer Risk Software
The U.S. Food and Drug Administration has granted De Novo authorization to ArteraAI Prostate, a multimodal artificial intelligence (MMAI) software developed by Artera, Los Altos, California headquartered precision medicine company, designed to assist clinicians with risk-based decisions in localized prostate cancer. The decision follows the product’s earlier Breakthrough Device Designation and allows its use as a regulated medical device in qualified pathology laboratories.
The FDA’s Breakthrough Devices Program supports the development of devices that may offer more effective diagnosis or treatment for serious conditions while maintaining standard safety and performance requirements. ArteraAI Prostate is claimed to be the first AI-powered risk stratification tool for prostate cancer to receive this designation.
Artera’s multimodal artificial intelligence (MMAI) platform combines digitized pathology slides with clinical data to deliver predictive and prognostic insights for personalized cancer care. Built with diverse patient cohorts, including representative African American populations, it preserves tissue by using existing biopsy slides and provides results within days. The system is designed to augment, not replace, physicians, reducing uncertainty in treatment benefit and enabling AI biomarker development across cancer types.
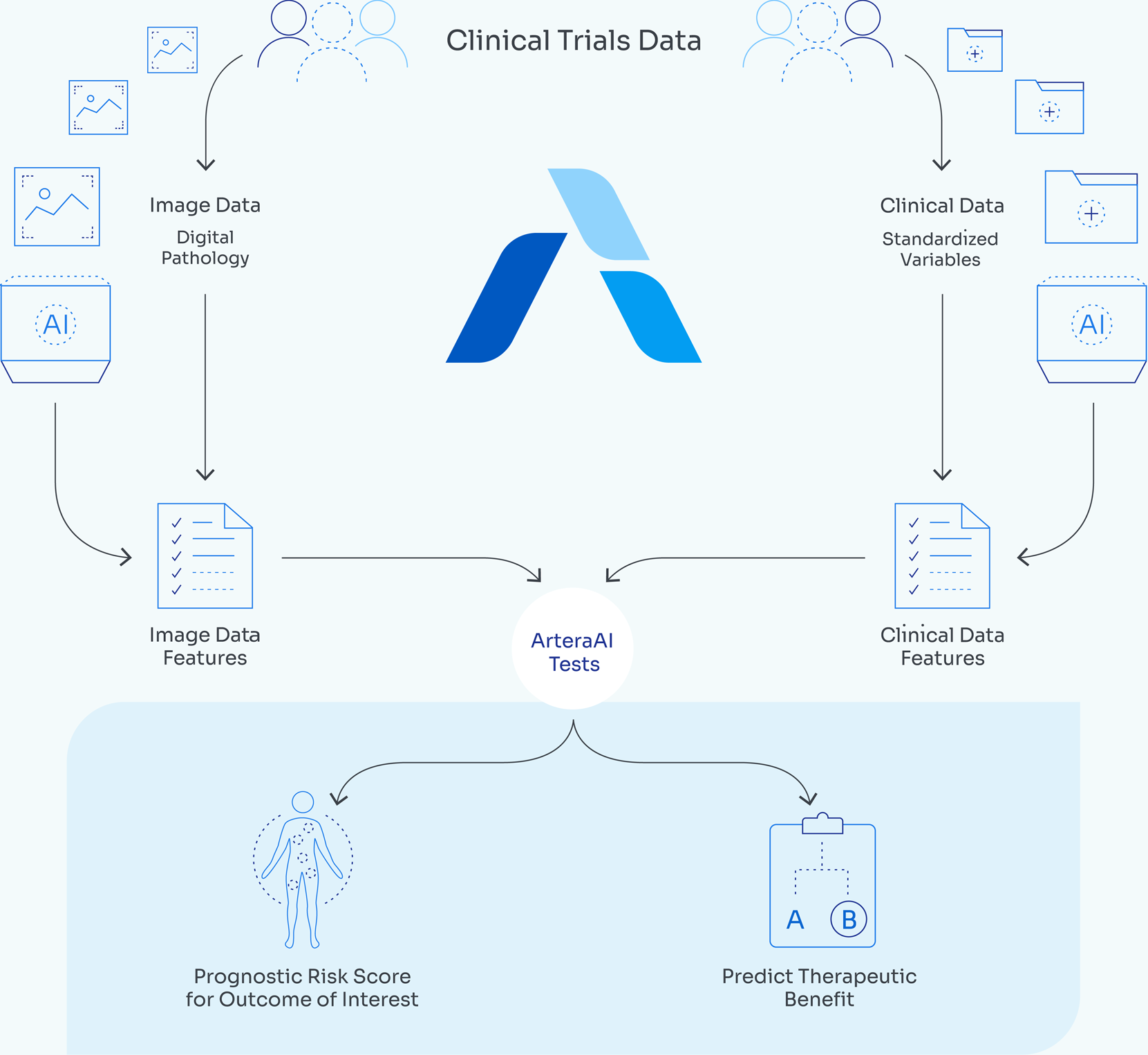
Image credit: ArteraAI in Cancer Therapy
ArteraAI Prostate analyzes digitized prostate biopsy images along with clinical data to estimate long-term outcomes, including 10-year risk of distant metastasis and prostate cancer–specific mortality. It can also assess potential benefit from specific therapies, such as androgen deprivation therapy or abiraterone when combined with radiation. The system is designed for use across all National Comprehensive Cancer Network (NCCN) prostate cancer risk groups, with output available within one to two days of receiving digitized biopsy images and patient data.
Image credit: ArteraAI Prostate Test
According to Artera, the platform’s prognostic and predictive models were developed using datasets from thousands of patients and tens of thousands of pathology slides, validated in multiple Phase 3 randomized trials with up to 15 years of follow-up.
Image credit: ArteraAI Prostate Test
The test is currently commercially available as a laboratory-developed test (LDT) through Artera’s Clinical Laboratory Improvement Amendments (CLIA)–certified, College of American Pathologists (CAP)–accredited lab in Jacksonville, Florida, and is listed in the NCCN Clinical Practice Guidelines for prostate cancer.
Topic: AI in Bio