Eli Lilly Signs up to $950M AI-Guided Cancer Therapeutics Deal with InduPro
Eli Lilly has signed a multi-target discovery agreement with InduPro, a U.S.-based biotech focused on multispecific therapeutics, to pursue new cancer treatments using an AI-enabled membrane interactomics platform. The deal includes up to three undisclosed oncology targets and carries a potential value of up to $950 million in milestones and upfronts, alongside an undisclosed equity investment by Lilly.
InduPro will lead early-stage discovery using its MInt (Membrane Interactomics) platform, which integrates machine learning to map spatial relationships of membrane proteins on tumor cells. This is intended to identify disease-specific target pairs for use in bispecific antibody-drug conjugates and multispecific T-cell engagers, with a focus on improving tumor selectivity and minimizing off-target toxicity.
InduPro is developing more tumor-selective therapeutics by discovering tumor-associated proximity antigens (TAPAs), surface proteins found in close proximity to known tumor-associated antigens (TAAs) within the tumor microenvironment. By co-targeting TAPAs and TAAs, the company aims to bypass limitations of current approaches that rely on TAAs alone, many of which are also expressed on healthy tissue, leading to safety challenges. This dual-targeting model is intended to enable selective therapeutic engagement of tumor cells while minimizing damage to normal cells.
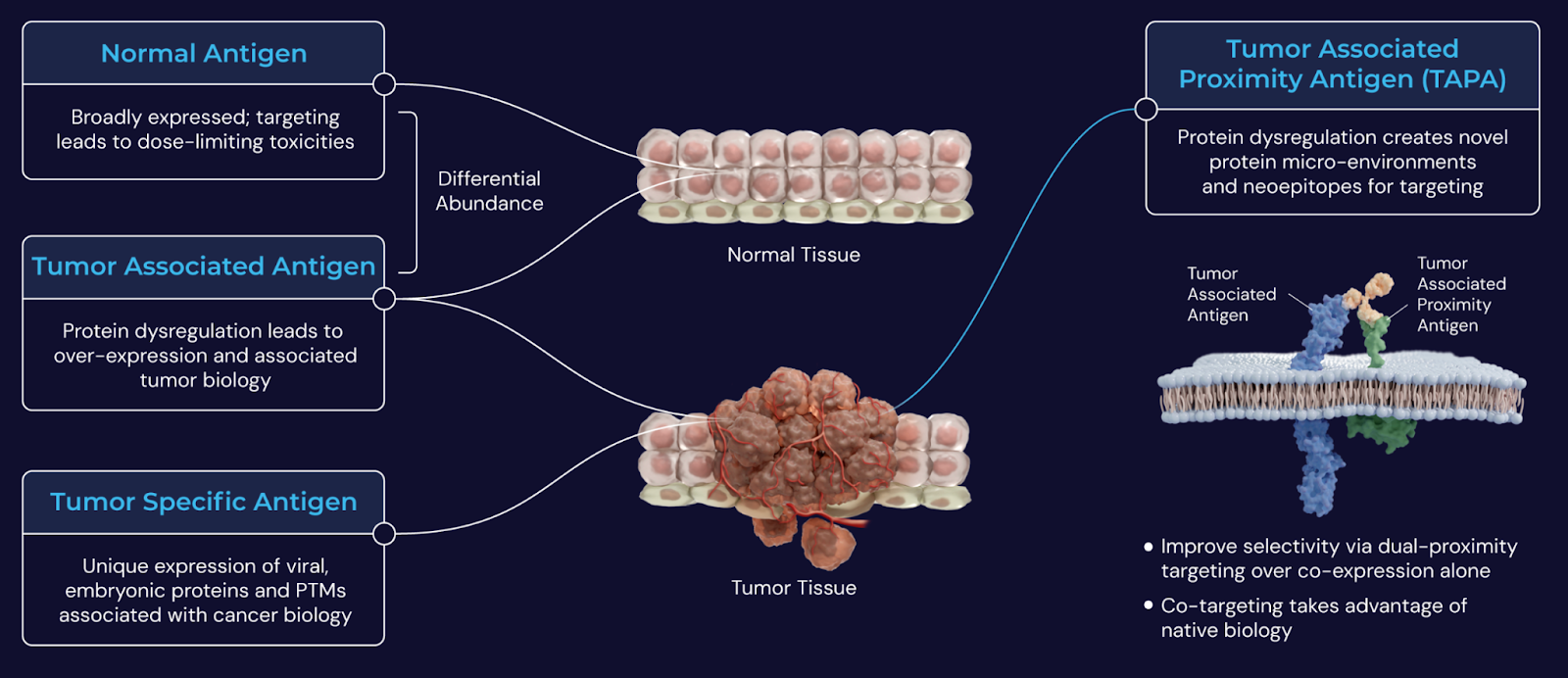
Image credit: InduPro
InduPro also employs an "induced proximity" strategy through its Binder Toolbox, which allows for artificial spatial reconfiguration of surface receptors to create or disrupt signaling pathways.
The company was founded in 2022 and operates out of Seattle and Cambridge, led by CEO Prakash Raman, PhD, who joined the company in 2024 after previously holding senior roles at Flagship Pioneering, Novartis, and AstraZeneca. InduPro’s latest disclosed financing was an $85 million Series A round in mid-2024.
Its therapeutic pipeline spans oncology and autoimmune indications, including a lead IDP-003, an engineered antibody that switches overactive immune cells off without killing them, in preclinical development for autoimmune diseases. It links two targets that naturally sit close together on T cells—PD1 and T-Cell Antigen 2—so that PD1 is pulled into the immune “contact zone” where signals to calm the cell are strongest, and it does this without needing the usual partner molecule PDL1.
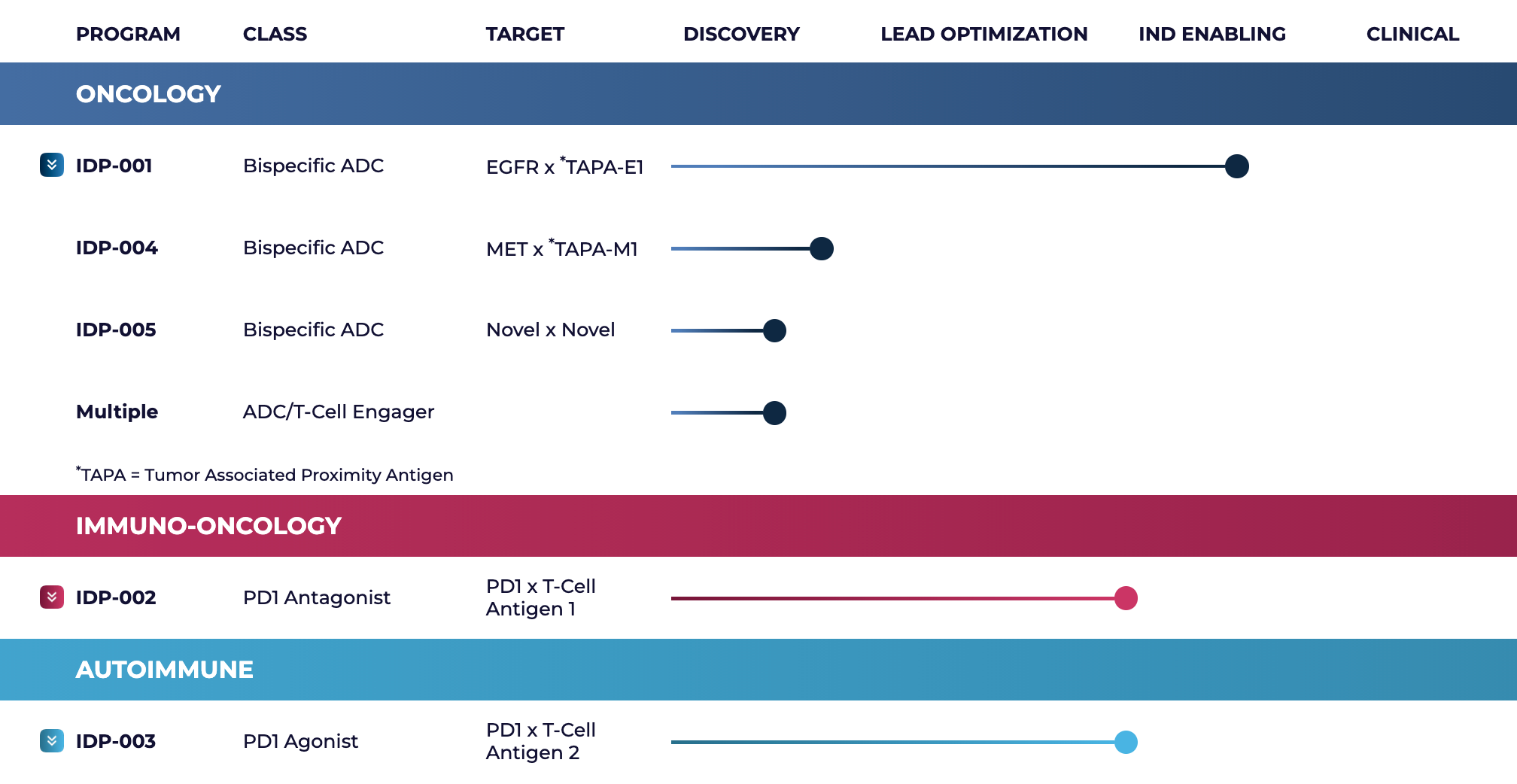 Image credit: InduPro’s pipeline
Image credit: InduPro’s pipelineThis is InduPro’s second publicly announced collaboration with a major pharmaceutical company within a month. In December 2025, the company announced a separate preclinical collaboration with Sanofi targeting bispecific PD-1 agonist antibodies for autoimmune conditions. IDP-001 is an experimental cancer drug that combines an antibody with a cell-killing payload and is built to recognize two markers on tumor cells at once: EGFR and a second tumor-specific antigen TAPA-E1.
In preclinical models, this dual-targeting approach reportedly has shown strong tumor-killing activity across several cancer types, and the company plans to file an IND to begin human testing in Q1 2026.
Topic: Industry Movers