BioAge Preps Brain-Penetrant NLRP3 Inhibitor for Obesity IND
BioAge Labs has finished the pre-clinical package for BGE-102, an orally available, brain-penetrant NLRP3 inhibitor that the company is advancing for obesity, and plans to submit an Investigational New Drug application in mid-2025.
In diet-induced obese mice, once-daily BGE-102 produced dose-dependent weight loss of roughly 15 percent—similar to semaglutide—and improved insulin sensitivity over four weeks; when combined with semaglutide the regimen achieved more than 20 percent weight reduction.
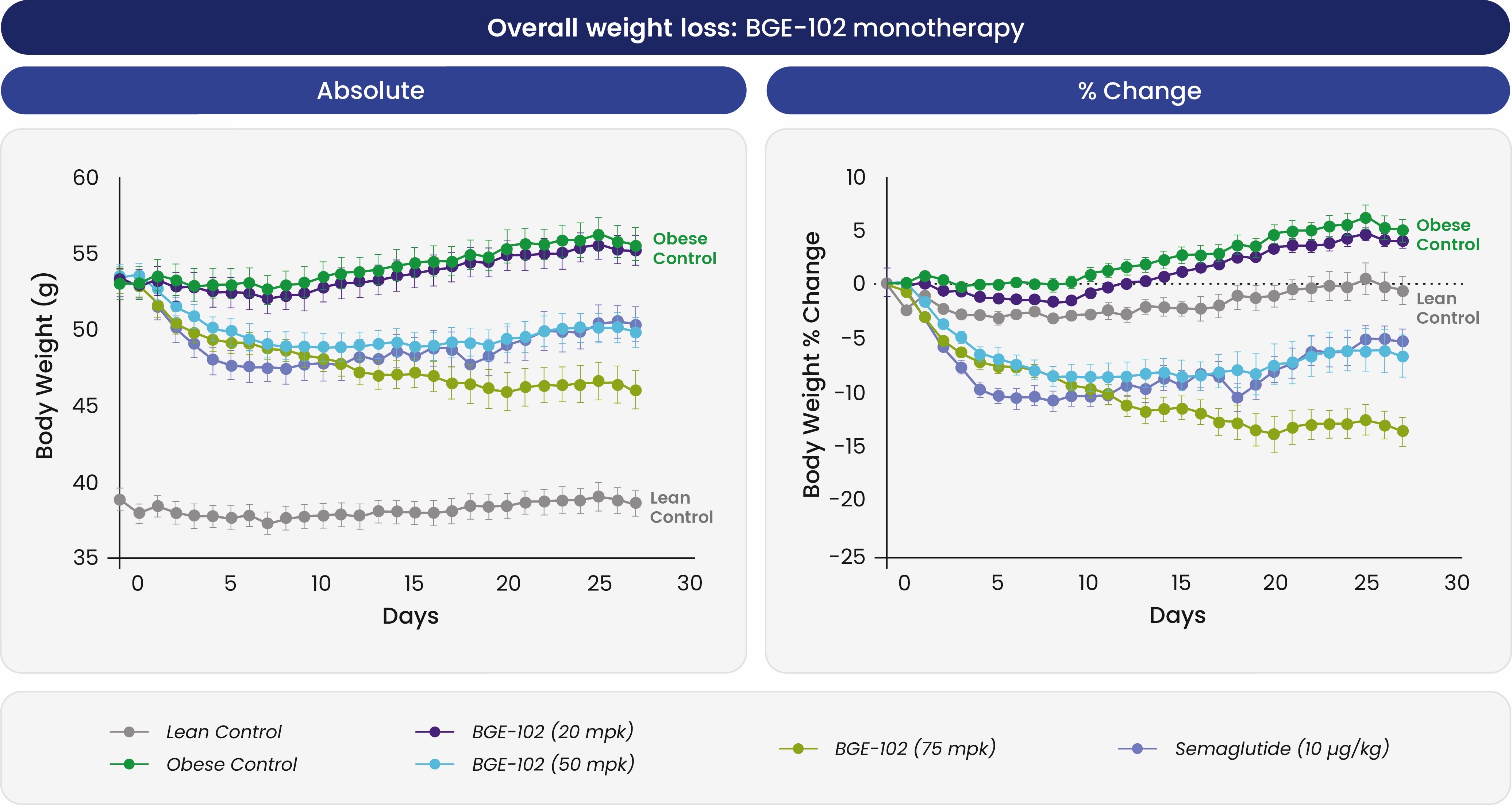
BGE-102 reportedly produced significant, dose-dependent weight loss in obese mice, with predicted human dosing below 50 mg due to higher potency in human microglia.
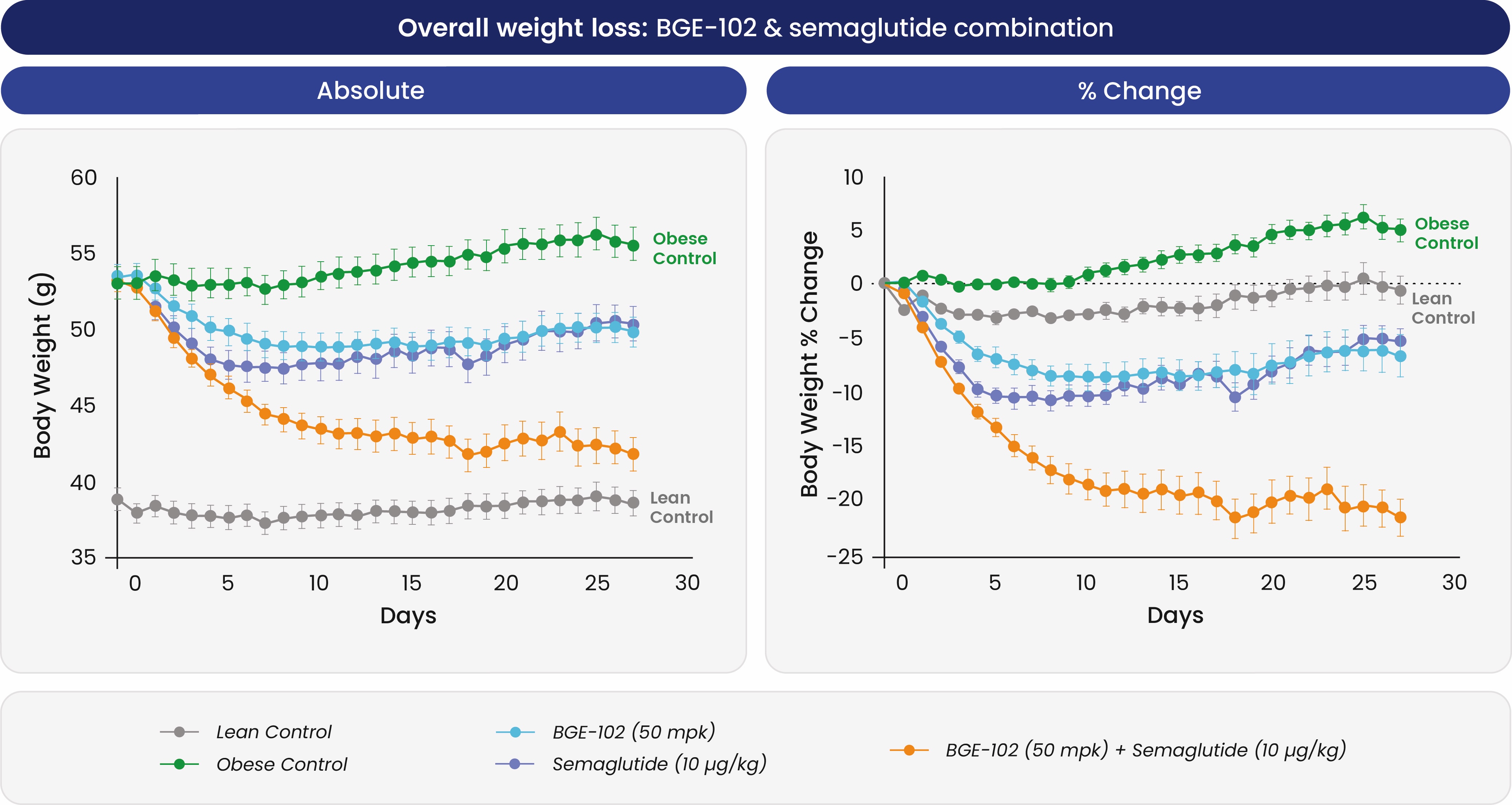
BGE-102 combined with semaglutide led to additive weight loss in obese mice, supporting potential use as a complementary therapy at low human doses.
The molecule binds a novel site on NLRP3, shows an IC90 of 2 nM in human microglia, and reaches a brain-to-plasma ratio of ~1. BioAge projects that these pharmacodynamics and a 35–75 × toxicology margin could support a once-daily oral dose below 50 mg. After IND clearance, the company expects initial single-ascending-dose data by year-end 2025 and plans a Phase 1b obesity study in the second half of 2026.
“By inhibiting the NLRP3 inflammasome, BGE-102 targets a core pathway that links metabolism, inflammation, and aging,” said BioAge co-founder and CEO Kristen Fortney.
BioAge’s discovery platform, which mines longitudinal aging cohorts, flagged lower NLRP3 activity as a correlate of longevity and guides additional programs, including pre-clinical APJ agonists for metabolic disease.