Aera Reports Preclinical Data on Lipid Nanoparticle-Delivered In Vivo CAR-T Therapy
Aera Therapeutics has presented preclinical results and nominated AERA-109, its first development candidate, for B cell–mediated autoimmune diseases. The candidate is built on the company’s targeted lipid nanoparticle (tLNP) delivery platform and is designed to generate CAR-T cells directly inside the body, avoiding conventional ex vivo manufacturing. Clinical trials are planned to begin in mid-2026.
AERA-109 is an in vivo CAR-T therapy aimed at treating B cell–driven autoimmune diseases. It uses Aera’s targeted lipid nanoparticle (tLNP) system to deliver genetic instructions that reprogram immune cells directly inside the body. Aside from AERA-109, the company’s pipeline includes candidates for autoimmune, cardiac, muscle, and central nervous system diseases at varying stages of development.
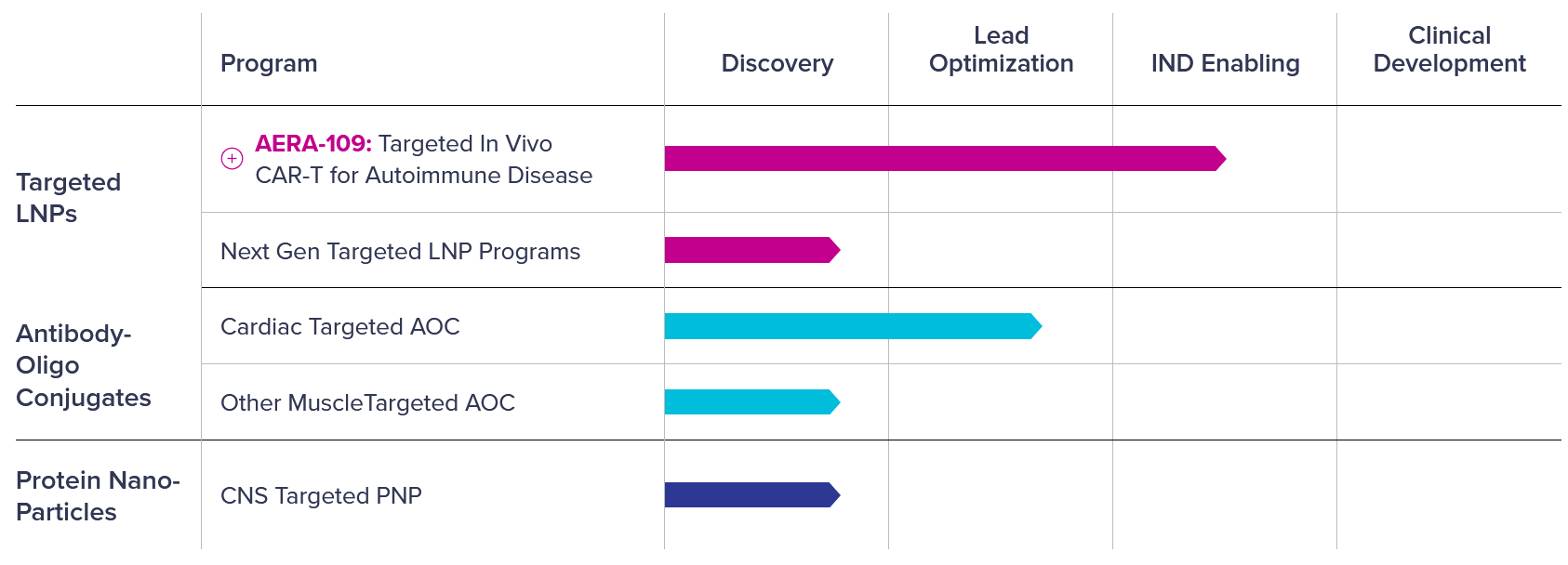
Image credit: Aera Therapeutics pipeline
At the 10th Annual CAR-TCR Summit, the company reported that AERA-109 produced dose-dependent CAR-T cell generation and deep B cell depletion in blood and tissues of humanized mouse models and non-human primates. Unlike traditional CAR-T therapies, the approach does not require lymphodepletion, viral vectors, or complex production steps.
Aera Therapeutics, headquartered in Cambridge, Massachusetts, is developing several delivery platforms aimed at making genetic medicines work in more tissues and disease settings:
- Targeted lipid nanoparticles (tLNPs): Built from proprietary ionizable lipids, designed to move beyond the liver-focused role of standard LNPs (e.g., vaccines) by reaching additional cell types.
- Antibody–oligonucleotide conjugates (AOCs): Combine antibodies, which guide drugs to specific tissues, with short DNA or RNA strands as therapeutic cargo. This design could extend antibody use to areas such as heart or muscle diseases.
- Protein nanoparticles (PNPs): Synthetic, cell-free particles made from engineered proteins that can be tuned to carry different genetic medicines. They circulate longer in the body and can be equipped with targeting ligands to reach chosen tissues.
We track developments like this weekly in Where Tech Meets Bio—our newsletter on startups, platforms, and deals at the intersection of biotech and digital.
Topic: Next-Gen Tools