1910 Releases AI Model That Designs Cell-Permeable Macrocyclic Peptides
Boston-based 1910 (previously 1910 Genetics) has introduced a new AI model, PEGASUS, designed to create a class of drug molecules—macrocyclic peptides—that are notoriously difficult to engineer for use inside the body. Published in the Journal of Medicinal Chemistry, PEGASUS generated reportedly the first known macrocyclic peptides with more than two polar or charged chemical groups that still show measurable cell permeability in lab tests. This addresses a long-standing challenge in peptide drug development—designing molecules that retain favorable drug-like properties, such as polarity or charge, while still being able to enter cells.
Founded in 2018, 1910 launched with a $4 million seed round led by OpenAI’s Sam Altman, later raising $26 million in a combined Seed and Series A funding backed by M12-Microsoft’s Venture Fund, Playground Global, OpenAI’s Sam Altman, and other leading investors. In 2024 the company entered a five-year commercial agreement with Microsoft to integrate its AI drug discovery platforms with Azure Quantum Elements.
Macrocyclic peptides are promising drug candidates because they can be more selective and potent than traditional small molecules. Most AI models avoid these kinds of peptides because there’s little data available to train on, especially for those with the chemical features needed to resemble approved drugs. 1910 developed PEGASUS to overcome this data problem.
The company built a large experimental dataset by testing 2.7 billion peptides in a lab system that mimics cell entry. This was combined with computer simulations and pattern-recognition techniques that help the model learn which structural features make a peptide more likely to be absorbed into cells.
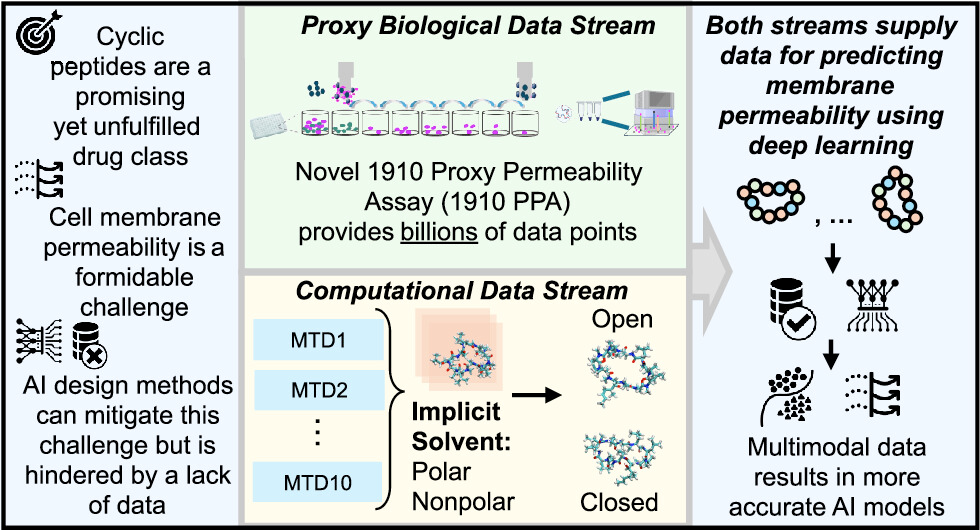
Image credit: PEGASUS: Unlocking Polarity in Cell-Permeable Cyclic Peptides Using AI Models Built on Massively Parallel Biological Assays
The system includes a generative model, CycPepVAE, which produced 33 candidate peptides resembling FDA-approved drugs in polarity and charge. Four of these were synthesized and shown to be permeable in vitro, suggesting oral bioavailability even with higher polarity profiles.
In November 2025 Journal of Medicinal Chemistry publication, 1910 also described CANDID‑CNS—an AI model that predicts whether small molecules can cross the blood–brain barrier. The work focuses on Beyond Rule‑of‑5 (bRo5) chemical space—compounds that fall outside conventional drug‑like property limits and are generally excluded from traditional design strategies, and incorporates stereochemistry, which refers to how the three‑dimensional arrangement of atoms affects biological behavior.
PEGASUS is part of 1910’s broader platform, ITO, which combines large-scale biological and chemical data, advanced AI models, and automated lab systems to accelerate the discovery of new drug targets and the design of both small and large molecule therapeutics.
Topic: AI in Bio