From Animals to Algorithms: How AI Brings Drug Testing Closer to Human Biology
Preclinical and nonclinical testing of drugs has traditionally relied on animal models to assess efficacy and toxicity before moving into human trials. However, these models raise ethical concerns and are costly and time-consuming. Most importantly, animal models often have low predictive power for the behavior of novel treatments in humans.
At least in part, this translational gap contributes to the high failure rates (averaging around 90%) of drugs that enter clinical development and raises fundamental questions about the role of animal experiments in drug development. Ethically, animal testing is arguably justifiable only if it can meaningfully predict outcomes in humans. Financially, committing millions of dollars and years of development to models with limited informative value is equally hard to defend.
As governments and regulators, such as the US FDA, push to reduce animal testing, researchers from academia, pharma, and biotech are increasingly turning to new approach methodologies (NAMs) for preclinical and nonclinical testing. Such NAMs have the potential to spare animal lives and replicate human biology more faithfully than animal models, and to do so more rapidly than traditional preclinical development (Figure 1). They include the use of advanced cultures of human cells and tissues, organ-on-a-chip technology, and computational methods such as artificial intelligence and machine learning (AI/ML) approaches. In particular, AI-based approaches offer a scalable alternative to animal testing that can make predictions based on a variety of data sources to provide a better understanding of human disease biology.
Figure 1
The Limits of Animal Models – and Why We Need Alternatives
While animal testing has long been seen as an invaluable step to reduce human risk and support hypotheses before human trials, animal models often fail to predict toxicity and efficacy in humans. For example, rodent models have been shown to be poor predictors of cancer drug toxicity and of idiosyncratic drug-induced liver injury. Animal models can also fail to predict immune reactions in humans, for example, in response to antibodies or gene therapies. In a 2006 trial of the CD28 superagonist TGN1412, which had been shown to be safe in nonhuman primates, six healthy volunteers developed a life-threatening cytokine storm.
A key problem with using animal models for efficacy prediction is that most laboratory animals do not spontaneously develop the diseases that researchers want to study. Researchers have to induce proxy disease states that cannot replicate the etiology and physiology of a naturally developing disease. Consequently, these models have low predictive power for diseases such as Alzheimer’s disease or for human tumor biology and tumor–immune microenvironment. For many rare, multisystem disorders, available animal models are either highly reductionist or unavailable altogether.
In line with the 3Rs in animal research (Replacement, Reduction, and Refinement), regulators and researchers are now pushing for new approach methodologies (NAMs). In vitro models, such as advanced cell-based systems, 3D organoids, and organ- or patient-on-chip platforms, are promising alternatives to animal models. This is especially promising in the rare disease space. For example, experiments in patient-specific, iPSC-derived organoids or chips could help address the heterogeneity of disease phenotypes caused by different mutations. They could even enable “clinical trials in a dish”, testing patient-specific efficacy and safety before exposing rare disease patients to novel treatments. However, such models are still laborious to implement and have several limitations. They are generally reductionist and do not yet capture multiorgan physiology, which is required to understand the biology of systemic diseases. Complementing cell- and organoid-based NAMs with other human-relevant approaches, such as AI models that learn on large, diverse datasets, can help capture a more comprehensive snapshot of human biology.
How AI Expands the NAM Toolkit
AI- and ML-based approaches are increasingly penetrating into pharma and biotech. While we have not yet seen the full-blown revolution some advocates predicted, there is a palpable shift toward using AI models for a wide range of tasks in drug discovery and development. Notably, many of these tasks are expected to reduce or eventually replace animal testing—for example, AI-driven in silico toxicology models that flag hepatotoxicity or cardiotoxicity before any in vivo studies, ML models that predict ADME properties and safe starting doses in humans, or algorithms that learn from organoid and organ-on-chip data to anticipate responses in human-relevant contexts (Figure 2).
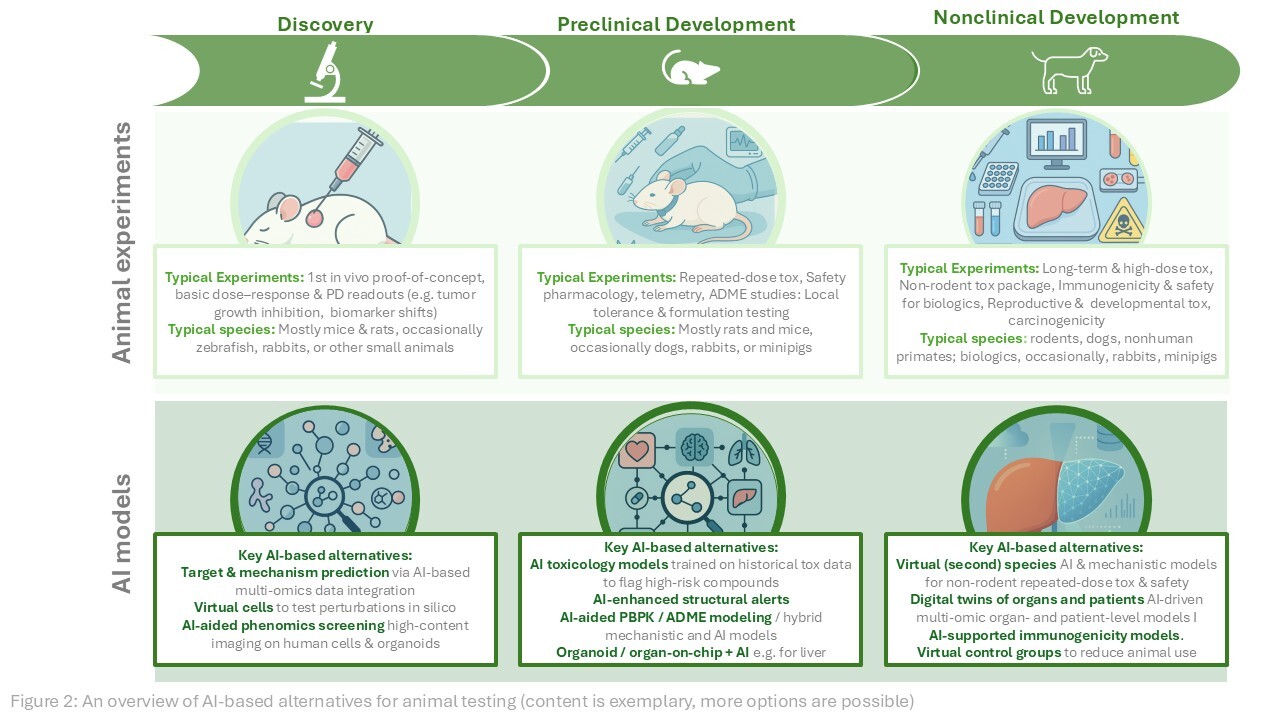
Figure 2
Compared with animal testing, AI models offer several advantages: they scale better and, once trained and validated, can be applied to millions of compounds, allowing researchers to explore more molecules, doses, and scenarios than would ever be feasible in vivo. They can also integrate diverse data types, such as chemical structures, various clinical endpoints, and omics readouts, into a single prediction, greatly enhancing their predictive power. Perhaps most importantly, AI models can be trained on human-relevant data, helping to bridge the long-standing translational gap.
However, the accuracy of AI-based NAMs is limited by the lack of high-quality human training data and by biases toward well-studied compounds, disease contexts, and patient groups. Incorporating data from organoid and chip-based systems and curating historical datasets can help build an important bridge toward human-relevant data. Another key challenge in using AI-based NAMs to replace animal tests lies in the “black-box” nature of many models. Explainable AI approaches—for example, a deep learning toxicity-prediction model developed by IBM researchers in 2023—will likely pave the way toward better acceptance of AI-based NAMs by researchers and regulators.
AI for Toxicity, ADME, and PBPK
Drug-induced toxicity that only becomes apparent in late-stage trials or even post-marketing creates substantial health risks for patients and financial risks for developers, as exemplified by drugs withdrawn because of cardiovascular events, such as rofecoxib (Vioxx), or liver failure, such as the NSAID bromfenac. By learning from millions of historical data points rather than from a few dozen animals in a single study, AI-based models can flag toxicity, including rare, subtle, or timing-dependent events.
One example of an AI model for toxicology prediction is the AnimalGAN model developed by the FDA, which uses data from TG-GATEs to generate synthetic clinical pathology data. AnimalGAN was trained on data from 6,442 rats across 1,317 treatment conditions and outperformed several traditional QSAR approaches. The synthetic data that the model produced correlated strongly with real measurements, even predicting rare events such as idiosyncratic drug-induced liver injury (DILI) while providing insights into mechanisms like liver necrosis.
The goal of reducing animal testing for toxicology fuels several large-scale, multi-stakeholder initiatives. For example, the Virtual Second Species project led by esqLABS aims to create a computational, organ-resolved “virtual dog” (with £1.6 million funding). Similarly, the EU project ONTOX (funded with €17.2 million) seeks to replace systemic repeated-dose animal tests with ontology-driven, AI-based NAMs for liver, kidney, and the developing brain.
A number of biotechs are investing in training AI for toxicity prediction on human data from well-curated clinical datasets, cell, organoid, or organ-on-chip data. For example, Quris-AI’s Bio-AI Clinical Prediction Platform combines “patients-on-a-chip” technology with ML algorithms to predict human drug safety before first-in-human studies. German pharma company Merck KGaA has piloted and integrated Quris’ platform after studies showed improved liver toxicity prediction versus traditional preclinical methods. Similarly, AI-driven startup Axiom, which launched with a seed round of $15 million in early 2025, has assembled a massive, well-curated training dataset including images of nearly 400 million liver cells exposed to over 115,000 chemicals, which are linked to clinical liver toxicity data with over 10,000 annotated compounds with liver-relevant endpoints. Axiom’s AI liver model has improved sensitivity for detecting relevant liver toxicity compared to similar models from pharma players.
Beyond toxicity prediction, AI is also entering the ADME and PBPK space. Predictions of absorption, distribution, and dose modeling have traditionally relied on mechanistic modeling. Recently, companies that offer computational ADME and PBPK solutions, such as Certara, VeriSIM Life, Optibrium, and esqLABS, increasingly invest in hybrid strategies that fuse AI/ML with mechanistic modeling and offer AI-based consulting services for preclinical and nonclinical drug testing.
Digital Disease Models and the Virtualization of the Lab
A number of biotech / techbio companies are developing AI-based models that can deepen disease understanding and test hypotheses for new compounds, either via in silico experimental systems or in AI-assisted tissue- or organ-on-chip platforms.
Aitia’s Gemini digital twins use multi-omic patient data to reconstruct causal disease networks and run billions of virtual perturbation experiments to identify druggable nodes. Aitia has multiple biotech and pharma collaborations across neurology and oncology, including with UCB, Servier, and Orion. Similarly, CytoReason builds AI-enabled disease models, which holistically integrate various data sources from clinical and omics data. Beyond its five-year, up-to-$110M deal with Pfizer, CytoReason raised a further $80M in 2024 from investors including NVIDIA, Pfizer, and Thermo Fisher to scale its platform.
French techbio NETRI offers a Neuron-as-a-Sensor organ-on-chip suite. The platform combines human iPSC-derived neurons on compartmentalized chips with ML-based analytics to build electrophysiological “digital signatures”. These signatures help to predict safety and efficacy for various compounds across indications such as pain, dermatology, and neurotoxicity. US biotech Vivodyne’s robotics-enabled platform of complex human tissues allows AI-based analysis of rich datasets, including for example secretory sampling, 3D confocal imaging for single-cell phenomics, and various omics endpoints. The company received $40 million in Series A in 2025, which will go into funding a fully robotic 23,000-square-foot lab.
AI- and robotics-driven platforms are beginning to scale into “lab-in-the-loop” and “virtual lab” paradigms, such as Recursion’s AI-driven phenomics platform that centers on its OpenPhenom-S/16 foundation model and TurbineAI’s virtual lab. Platforms that use AI to replace or better target animal experiments to contexts where high predictive value is expected, which are likely to improve disease understanding and clinical success rates, while decreasing preclinical development time in the long run.
Outlook – What It Takes to Make AI-Based Alternatives the New Normal
Momentum for alternatives to animal testing is building, with regulators and innovators pushing in the same direction. After the FDA Modernization Act 2.0 was signed in 2022, in 2025 the agency moved to let NAMs (including AI-driven toxicology models) stand in for many animal tests for monoclonal antibodies. The agency stated that over the next 3-5 years animal studies should become “the exception rather than the norm for pre-clinical safety/toxicity testing”. While the regulatory shift is not as clearly spelled out by the EMA, the EU now accepts 3R-compliant NAMs within the regulatory lifecycle and is rolling out a roadmap toward phasing out animal testing for chemical safety assessments. This mandates regulatory guidance for the acceptance of such models, such as a clearly defined context-of-use, robust validation, and interlaboratory reproducibility. It will also require an alignment on standards and frameworks to ensure that a NAM is at least as effective as the animal test it replaces to protect patient safety, for example through the multidisciplinary Validation and Qualification Network, initiated by the NIH. To navigate regulatory uncertainty around NAMs, several companies focused on computational alternatives to animal testing, such as VeriSIM, Certara, and SimulationPlus, are helping pharma partners navigate the shift from animal models to NAMs. Their offerings include toolkits like Certara’s Non-Animal Navigator™ and Simulations Plus’s NAMVantage™, which are designed to support the adoption and integration of non-animal methods. The need for alternatives to animal testing is also recognized by players focused traditionally on animal experiments, such as the CRO Charles River. The company has dedicated around $500M for its Alternative Methods Advancement Project (AMAP) and is increasingly using AI-enabled NAMs to reduce reliance on animal testing for example, by mining large historical datasets and running virtual experiments.
At the same time, AI-first biotechs are beginning to demonstrate that cell, organoid or chip-based, or fully virtual evidence can shrink preclinical animal use and accelerate development timelines. The upfront investment for NAMs can be higher than simply continuing with animal studies—and some platforms, such as organs-on-chips, may not be cheaper than rodent tests—but if they deliver on their core promise of better translation to humans, the long-term scientific, ethical, and economic payoff could be enormous.
References
-
https://pubmed.ncbi.nlm.nih.gov/39805539/
-
https://www.nature.com/articles/s41587-025-02690-0
-
https://www.nature.com/articles/d41586-025-03344-6
-
https://pubmed.ncbi.nlm.nih.gov/35865092/
-
https://pubmed.ncbi.nlm.nih.gov/32868897/
-
https://pubmed.ncbi.nlm.nih.gov/32324077/
-
https://pubmed.ncbi.nlm.nih.gov/18357347/
-
https://pmc.ncbi.nlm.nih.gov/articles/PMC9100373/
-
https://pubmed.ncbi.nlm.nih.gov/31291566/
-
https://aacrjournals.org/cancerdiscovery/article/8/9/1069/10253/Fundamental-Mechanisms-of-Immune-Checkpoint
-
https://www.nature.com/articles/s41746-025-02068-1
-
https://pubmed.ncbi.nlm.nih.gov/33356151/
-
https://pubmed.ncbi.nlm.nih.gov/39836754/
-
https://pubmed.ncbi.nlm.nih.gov/37676606/
-
https://www.frontiersin.org/journals/artificial-intelligence/articles/10.3389/frai.2023.1269932/full
-
https://www.nature.com/articles/s41598-023-31169-8
-
https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/vioxx-rofecoxib-questions-and-answers
-
https://onlinelibrary.wiley.com/doi/10.1002/pds.1207
-
https://www.nature.com/articles/s41467-023-42933-9
Topic: Next-Gen Tools