AI and CRISPR—converging revolutions in biomedicine?
Since the discovery of its mechanism in 2012, CRISPR-based gene editing has turned from basic discovery into a bioengineering tool now penetrating biomedicine and enabling breakthroughs in the treatment of rare diseases. The first approved gene editing therapy, Vertex’s Casgevy, is an ex vivo therapy for rare hematological disorders such as sickle cell disease and β-thalassemia that received regulatory approval in 2023, with many other programs following in clinical development.
However, while CRISPR allows researchers to make precise edits in the genome with relative ease, the success of CRISPR experiments remains constrained by inefficiencies and imprecision and requires high skill levels. Moreover, CRISPR-based therapies are still highly individualized and high-priced, and have not yet reached their potential as a platform technology for curing rare diseases.
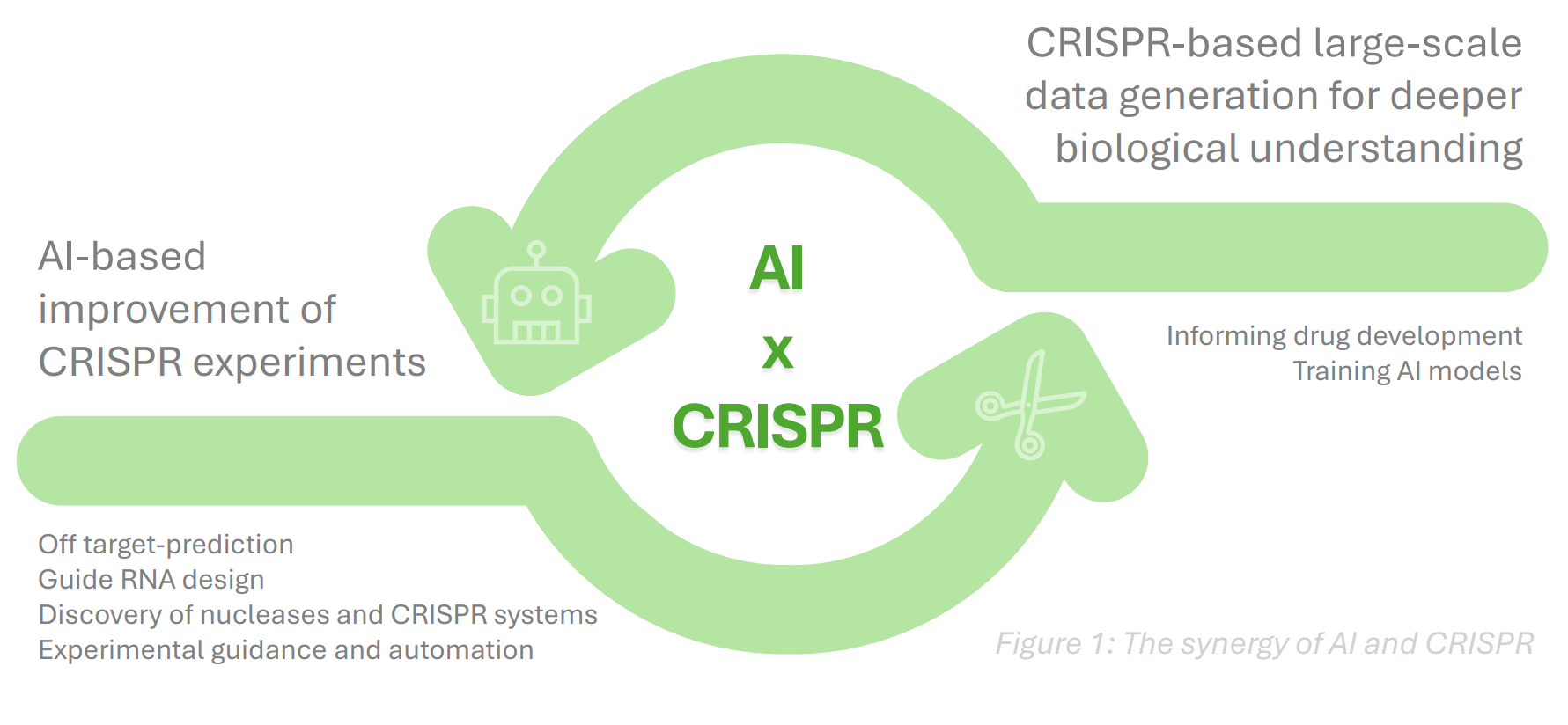
A recent milestone is the AI-agent system CRISPR-GPT, which aims to lower the expertise threshold for planning and executing CRISPR experiments. In the future, such agentic systems could feed into large-scale, CRISPR-based data-generation initiatives at biotechs like Xaira and Recursion. In this way, a virtuous cycle could emerge. CRISPR experiments that elucidate human biology would train AI/ML models, which in turn would optimize and scale CRISPR workflows, closing the automation loop and potentially reducing CRISPR costs.
The promise and challenges of CRISPR-based gene editing
CRISPR–Cas is a genome-editing technology adapted from a bacterial immune system in which fragments of viral DNA from past infections are stored as short RNAs that guide a nuclease to cleave complementary sequences in an invading virus to disable it. Scientists have repurposed this mechanism for bioengineering, using CRISPR systems to edit genomes in the laboratory and, increasingly, in clinical contexts. Among the various CRISPR systems, CRISPR–Cas9, in which the Cas9 nuclease cleaves DNA, has become the most widely adopted because of its relative ease of use and cost-effectiveness.
However, the CRISPR–Cas9 system has several challenges. The size of Cas proteins can limit editing efficiency. Moreover, nucleases like Cas9 create double-strand DNA breaks, which—depending on the chosen DNA repair pathway—can produce insertions or deletions that lead to unwanted genomic edits and harmful changes. To improve precision and expand the repertoire of possible edits, newer CRISPR systems, such as base editing, prime editing and epigenetic editing, have emerged that don’t rely on Cas nuclease–induced double-strand breaks.
Base editors link deaminase enzymes to inactive Cas proteins to enable single-base transitions directly in DNA, a technique that holds promise for correcting disease-causing point mutations common in rare diseases. Prime editors, which couple a Cas9 nickase to a reverse transcriptase and use a pegRNA to introduce the desired change at the target site, hold promise for edits that other CRISPR systems cannot perform, with increased precision. A number of biotech companies are exploring these systems, such as base-editing company Beam Therapeutics and prime-editing company Prime Medicine.
However, alternative CRISPR systems also face challenges in efficiency and precision: base editors can produce bystander edits and context-dependent off-target effects, and prime editing often shows comparatively low efficiencies. Enhancing CRISPR efficacy and specificity will be essential for improving safety and effectiveness in gene-editing-based therapies. At the same time, novel CRISPR systems promise a wider range of editing use cases, which may enhance applicability of CRISPR technology in therapeutic contexts.
AI-aided CRISPR experiments
AI models can enhance CRISPR by optimizing guide-RNA (gRNA) design, predicting and reducing off-target activity, improving overall editing efficiency, and lowering the expertise threshold for researchers. Multiple academic groups, biotechs, and tech companies are customizing models to improve CRISPR precision, discover/design novel proteins, and streamline experiment design.
Improving targeting accuracy through guide RNA design and off-target prediction
A growing set of AI models have been developed to improve gRNA design and reduce off-target editing for CRISPR–Cas9, base, and prime editing systems. For example, the DeepCRISPR model, published in 2018, improves single-guide RNA design and enables both on- and off-target prediction.
The Broad Institute/Microsoft’s Azimuth (on-target) and Elevation (off-target) models together provide an end-to-end AI pipeline for sgRNA selection. Other approaches, such as a 2025 study from Foshan University, China, use large language models (LLMs) to design gRNAs with lower off-target potential. Their model, CCLMoff incorporates a pretrained RNA language model from RNAcentral, which captures sequence relationships between guide RNAs and their potential target sites.
AI discovers and designs new CRISPR proteins with higher efficiency
A promising route to improve CRISPR efficiency and accuracy is the discovery or design of novel editors with properties such as smaller size, reduced off-target activity, and lower immunogenicity.
For example, biotech company Profluent and the Arc Institute developed large-scale molecular language models, trained on vast amounts of diverse prokaryotic and phage genomes to design new CRISPR systems. Profluent researchers compiled a “CRISPR–Cas Atlas” of ~1.246 million diverse CRISPR–Cas operons to retrain an updated version of their ProGen protein language model. After fine-tuning their model towards Cas families, they generated millions of candidate Cas proteins and synthesized 209 AI-designed Cas9-like variants.
Their lead, OpenCRISPR-1, showed similar on-target efficacy as the commonly used SpCas9 protein from Streptococcus pyogenes, from which it differs by 403 mutations. Off-target edits were reduced by 95% for OpenCRISPR-1. Moreover, the AI-generated Cas nuclease did not introduce novel off-target patterns, and appeared potentially less immunogenic, not having known SpCas9 T-cell epitopes and binding less human antibody in vitro.
Profluent released OpenCRISPR-1 for free licensing for ethical research and commercial use. Going beyond nuclease design, the Arc Institute’s Evo model is among the first examples of protein–RNA and protein–DNA co-design with a language model, trained on millions of full prokaryotic and phage genomes to capture relationships across DNA, RNA, and proteins in long-context genomic settings.
Several biotechs, such as Mammoth Biosciences, Metagenomi, and Arbor Biotechnologies focus on AI-driven metagenomic discovery platforms to find and engineer new types of Cas enzymes and CRISPR systems. For example, Mammoth Biosciences’, ultracompact nucleases (e.g., Cas14, CasΦ) are easier to deliver for in vivo editing; and support both therapeutic and diagnostic tools. In 2024 Mammoth entered a partnership with Regeneron with a $100 million upfront payment for access to its ultracompact CRISPR-based gene editing platform.
Similarly, Metagenomi uses its AI platform for finding new nucleases with a focus on both liver and extrahepatic delivery. Shortly after Metagenomi’s 2024 public offering, Moderna walked away from a deal with Metagenomi, which was worth up to $3 billion, stating strategic reprioritization.
Cambridge-based biotech Arbor Biotechnologies uses AI/ML feedback loops with high-throughput screening and protein engineering to optimize their editors and reports more than 30-fold gains in editing efficiency over wild-type nucleases and the discovery of diversified CRISPR systems that promise greatly increased coverage of the human genome. In October 2025, Arbor struck a deal on gene editing for rare diseases with Chiesi, with an upfront of $115 million and up to $2 billion total deal value.
Epic Bio, is a company focused on epigenetic editing therapies using its Gene Expression Modulation System (GEMS). In 2023, the company reported a combination of high-throughput screening of hundreds of transcription-activating peptides with machine learning to predict the most potent combinations, enabling the design of multiplexed GEMS constructs with enhanced gene-expression activity.
AI models can also be used to boost the efficacy of advanced Cas systems. For example, Scribe Therapeutics’ CasX editor is a nuclease which is diverged from its naturally occurring counterpart, with 100x improvements in activity and no detectable off-target editing. In September 2025, the California-based biotech introduced its AI/ML platform DeepXE, a machine-learning model that predicts CasXE potency. According to the company presentation, the AI models doubled hit rates when compared to conventional models.
Table 1: Selected Biotechs combining CRISPR with AI
|
Company (HQ) |
Editing Modality |
Focus / Therapeutic Area |
AI usage |
Funding & Deals (recent) |
|
Base editing |
Hematology/ In-vivo liver-targeting |
Public (NASDAQ: BEAM); $500mn public offering (March 2025)
|
||
|
Mammoth Biosciences (Brisbane, CA) |
Ultra‑compact nucleases (CasΦ/Cas14); |
In‑vivo editing across multiple tissues: Diagnostics & therapeutics (Liver, neuromuscular, CNS) |
Private; Series D $150mn (Sep 2021) |
|
|
Scribe Therapeutics (Alameda, CA) |
Engineered CRISPR nucleases, editing & epigenetic silencing |
Cardiometabolic (PCSK9 LDL‑C lowering) CNS (Prevail partnered) |
DeepXE AI/ML platform for guide/ editor potency prediction |
Private; Series B $100mn (Mar, 2021), |
|
(Emeryville, CA) |
Novel CRISPR nucleases; extrahepatic delivery; targeted integration |
Liver, cell therapies for solid tumors, neuromuscular |
AI/ML to mine metagenomic database and discover editors |
Public (NASDAQ: MGX); IPO ~$94M (Feb 2024) Deal with Moderna worth up to $3B, ended by Moderna in May 2024 |
|
(Cambridge, MA) |
Diverse CRISPR editors (incl. Type V); precision knock‑in |
CNS, Liver, Autoimmune |
Private; Series C $73.9M (Mar 2025) Vertex (2018, 2021), Ginkgo Bioworks (2023) Acuitas (2023), |
|
|
Epic Bio (Epicrispr) South San Francisco, CA |
Epigenetic editing (GEMS; ultra‑compact Cas / epigenetic enzymes) |
Genetic muscular dystrophy |
Series B $68M (Mar 2025) |
|
|
(Emeryville, CA) |
Generative‑AI–designed gene editors |
Tools/platform; for designing editors / CRISPR systems |
Foundational generative AI for protein/gene‑editor design |
Series A $35M (Mar 2024) |
|
(Emeryville, CA) |
CRISPR-driven screens to generate data for drug development * |
Immunology / Precision oncology |
AlgenBrain: foundation model/ deep learning on single-cell CRISPR modulation data to map disease trajectories & target–outcome relationships |
Seed $9M (Feb 2022) |
*note that there are several other biotechs using similar approaches not listed here
CRISPR-GPT
A 2025 paper in Nature Biomedical Engineering introduced CRISPR-GPT, an LLM-based multi-agent system that acts as a mentor for researchers planning and conducting CRISPR experiments. The system was developed by researchers from Stanford, Princeton, Google DeepMind, and UC Berkeley and caused a stir in the scientific community and beyond due to its potential to lower the skill threshold required for CRISPR-based gene editing – a feat with potentially wide-ranging implications.
CRISPR-GPT combines general-purpose LLM reasoning with CRISPR-specific domain knowledge. Training data included scientific literature, public databases, and, interestingly, expert-curated discussions from a forum with more than 4,000 discussions of CRISPR troubleshooting, which the corresponding author Le Cong likened to a “Reddit for Gene Editing”. The type of scientific discussions likely mimics the scientific thought process of trial and error better than the polished output presented in scientific literature.
It orchestrates work through collaborating agents, including a user-proxy agent that interacts in natural language. A planner agent maps the workflow, selecting Cas proteins, guides, and delivery methods, while an executor agent designs gRNAs, invokes bioinformatics tools, drafts protocols, and analyzes data. Tool-provider agents connect to PubMed, Google Scholar, and other specialized CRISPR resources. To accommodate different expertise levels, CRISPR-GPT offers three modes: Meta Mode for beginners, Auto Mode for more experienced users, and Q&A Mode for targeted questions.
Notably, junior researchers with no prior gene-editing experience achieved high editing efficiencies on their first attempts when assisted by CRISPR-GPT. One researcher executed a four-gene knockout in A549 lung adenocarcinoma cells, achieving ~80% editing across all four targets. Another performed epigenetic activation of two immunotherapy-resistance genes in a melanoma cell line, with activation efficiencies of 56.5% and 90.2%, respectively.
However, the same capabilities that make AI-assisted CRISPR powerful also introduce risks if misused, from germline editing to pathogen design. CRISPR-GPT implements guardrails that block germline targets and high-risk viruses and addresses genomic privacy by avoiding storage of identifiable sequences and filtering sensitive inputs. Still, it remains an open question whether skilled users might circumvent such safeguards in CRISPR-GPT or other LLMs.
The promise and risks of AI-aided gene editing
Beyond gains in accuracy and efficiency, AI agents could help make gene editing more accessible by lowering barriers, guiding complex workflows, and automating design and analysis. This could help accelerate the development of lifesaving therapies for rare and genetic diseases, as was shown in the case of an infant with a rare, life-threatening disease who could be saved by personalized gene editing therapy in a Philadelphia hospital.
CRISPR and AI are enabling technologies, which individually and synergistically have the potential to reshape biomedical research and clinical medicine. Despite this potential (and the surrounding hype), both technologies are still at an early stage. A bit more than a decade into biomedical use of CRISPR (and even less for AI), the technologies currently often deliver small-scale improvements and individual breakthroughs rather than overall disruptive changes. It remains to be seen whether AI can help CRISPR to the next level, toward becoming a true platform technology that can offer such individualized cures for rare genetic diseases in a scalable fashion.
References
- https://www.nature.com/articles/s12276-025-01452-x
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8571165/
- https://www.nature.com/articles/s12276-025-01462-9
- https://www.nature.com/articles/s41551-025-01463-z
https://www.nature.com/articles/nature17946 - https://www.nature.com/articles/s41587-024-02320-1
- https://www.labiotech.eu/best-biotech/crispr-companies/
- https://www.labiotech.eu/in-depth/crispr-ai/
- https://pubmed.ncbi.nlm.nih.gov/32533916/
- https://genomebiology.biomedcentral.com/articles/10.1186/s13059-018-1459-4
- https://www.nature.com/articles/s42003-025-08275-6
- https://www.genengnews.com/topics/genome-editing/crispr-meets-gpt-to-supercharge-gene-editing/
- https://www.nature.com/articles/s42256-023-00739-w
- https://www.biorxiv.org/content/10.1101/2024.04.22.590591v1.full
- https://www.nature.com/articles/s41586-025-09298-z
- https://pubmed.ncbi.nlm.nih.gov/39541441/
- https://med.stanford.edu/news/all-news/2025/09/ai-crispr-gene-therapy.html
Topic: Next-Gen Tools