
Roman Kasianov
Covers AI in drug discovery, digital pathology, and translational R&D, editor of "Where Tech Meets Bio"; oversees company profiles, partnerships, and core operations at BiopharmaTrend.

Covers AI in drug discovery, digital pathology, and translational R&D, editor of "Where Tech Meets Bio"; oversees company profiles, partnerships, and core operations at BiopharmaTrend.

Merge Labs has emerged from stealth with $252 million in funding to develop non-implantable brain–computer interfaces that use ultrasound and molecular approaches rather than electrodes. The company is cofounded by Sam Altman and is positioned at the intersection of neurotechnology, …

Insilico Medicine reported the deployment of its Nach01 multimodal foundation model on Microsoft Discovery, Microsoft’s Azure-based platform for AI-driven research and development. The demonstration illustrates how third-party foundation models can be orchestrated within a single enterprise environment to support end-to-end …

The Novo Nordisk Foundation has committed up to €736 million (~$856 million) to Copenhagen-based BioInnovation Institute in a nine-year funding program aimed at expanding early-stage life sciences and deep tech activity across Europe. The capital is earmarked to increase the …

Converge Bio, a Boston- and Tel Aviv-based startup developing generative AI systems for drug discovery, has raised a $25 million oversubscribed Series A round led by Bessemer Venture Partners, with participation from TLV Partners, Saras Capital, Vintage Investment Partners, and …

Eli Lilly and NVIDIA announced at the January 12, 2026 J.P. Morgan Healthcare Conference in San Francisco that they are creating a new AI co-innovation lab in South San Francisco, with the companies planning to invest up to $1 billion …

Owkin has entered its first collaboration with NVIDIA to advance the development of its biological large reasoning model, OwkinZero, using NVIDIA’s open AI infrastructure and reinforcement learning toolkit.
Announced today at JPM Healthcare Conference 2026 in San-Francisco, the …

Insilico Medicine reported first patient first dose in a Phase IIa clinical trial of its gut-restricted PHD inhibitor ISM5411, now assigned the generic name garutadustat.
The milestone moves the AI-designed program into mid-stage clinical testing in ulcerative …

LinkedIn founder Reid Hoffman and Siddhartha Mukherjee’s Manas AI has entered a multi-year strategic agreement with Schrödinger, securing privileged access to its physics-based molecular modeling platform along with direct engagement from internal technical teams.
Announced during the 2026 …
Boltz has launched as a public benefit corporation and paired the company formation with three operational announcements: a $28 million seed financing, a multi-year collaboration with Pfizer, and a preview release of its hosted platform with two initial discovery …
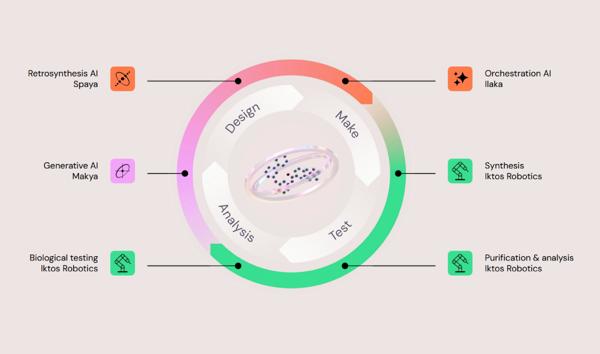
Iktos and Servier have entered a multi-year, multi-target research collaboration under which Iktos will apply its AI-driven molecular design and automated synthesis platform to discover small-molecule drug candidates, while Servier will lead biological evaluation and downstream development. The agreement spans …